 | |
| Clinical data | |
|---|---|
| Trade names | Evrysdi |
| Other names | RG7916; RO7034067 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.278.103 |
| Chemical and physical data | |
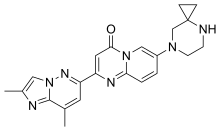
| Formula | C22H23N7O |
| Molar mass | 401.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Risdiplam, sold under the brand name Evrysdi, is a medication used to treat spinal muscular atrophy (SMA)[6][9] and the first oral medication approved to treat this disease.[6][9]
Risdiplam is a survival of motor neuron 2-directed RNA splicing modifier.[6][5][10]
In clinical trials, the most common adverse events included fever, diarrhea, rash, ulcers of the mouth area, joint pain (arthralgia) and urinary tract infections.[6][5] Additional adverse events observed in the infantile-onset population included upper respiratory tract infection, pneumonia, constipation and vomiting.[6][5]
Risdiplam was approved by the US Food and Drug Administration (FDA) in August 2020, for the treatment of adults and children two months of age or older.[6][11] Developed by Roche in Basel, Switzerland,[12] in association with PTC Therapeutics and the SMA Foundation,[9][11] it is marketed in the US by Genentech,[6] a subsidiary of Roche.[11]
- ^ a b "Evrysdi". Therapeutic Goods Administration (TGA). 11 June 2021. Retrieved 6 September 2021.
- ^ a b "AusPAR: Risdiplam". Therapeutic Goods Administration (TGA). 13 September 2021. Retrieved 13 September 2021.
- ^ "Summary Basis of Decision (SBD) for Evrysdi". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ "Health product highlights 2021: Annexes of products approved in 2021". Health Canada. 3 August 2022. Retrieved 25 March 2024.
- ^ a b c d Cite error: The named reference
Evrysdi labelwas invoked but never defined (see the help page). - ^ a b c d e f g h O'Keefe L (7 August 2020). "FDA Approves Oral Treatment for Spinal Muscular Atrophy" (Press release). Silver Spring, Maryland, United States of America: United States Food and Drug Administration (FDA). FDA Newsroom Department. Archived from the original on 11 August 2020. Retrieved 7 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Evrysdi EPAR". European Medicines Agency. 24 February 2021. Retrieved 4 March 2023.
- ^ "Evrysdi Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c Food and Drugs Administration (FDA) (7 August 2020). "Evrysdi (Risdiplam) for Spinal Muscular Atrophy". SMA News Today. Pensacola, Florida, United States: BioNews Services (BioNews Services, LLC.). Archived from the original on 27 January 2021. Retrieved 9 June 2021.
- ^ Cite error: The named reference
Zhaowas invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
Genentech PRwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Ratni Ebeling Baird Bendels pp. 6501–6517was invoked but never defined (see the help page).