| Robinson annulation | |
|---|---|
| Named after | Robert Robinson |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | robinson-annulation |
| RSC ontology ID | RXNO:0000380 |
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds.[1] The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
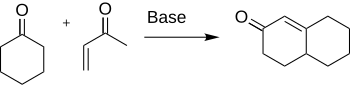
Formation of cyclohexenone and derivatives are important in chemistry for their application to the synthesis of many natural products and other interesting organic compounds such as antibiotics and steroids.[2] Specifically, the synthesis of cortisone is completed through the use of the Robinson annulation.[3]
The initial paper on the Robinson annulation was published by William Rapson and Robert Robinson while Rapson studied at Oxford with professor Robinson. Before their work, cyclohexenone syntheses were not derived from the α,β-unsaturated ketone component. Initial approaches coupled the methyl vinyl ketone with a naphthol to give a naphtholoxide, but this procedure was not sufficient to form the desired cyclohexenone. This was attributed to unsuitable conditions of the reaction.[1]
Robinson and Rapson found in 1935 that the interaction between cyclohexanone and α,β-unsaturated ketone afforded the desired cyclohexenone. It remains one of the key methods for the construction of six membered ring compounds. Since it is so widely used, there are many aspects of the reaction that have been investigated such as variations of the substrates and reaction conditions as discussed in the scope and variations section.[4] Robert Robinson won the Nobel Prize for Chemistry in 1947 for his contribution to the study of alkaloids.[5]
- ^ a b Rapson, William Sage; Robinson, Robert (1935). "307. Experiments on the synthesis of substances related to the sterols. Part II. A new general method for the synthesis of substituted cyclohexenones". Journal of the Chemical Society (Resumed): 1285. doi:10.1039/JR9350001285.
- ^ Heathcock, Clayton H.; Ellis, John E.; McMurry, John E.; Coppolino, Anthony (1971). "Acid-catalyzed Robinson Annelations". Tetrahedron Letters. 12 (52): 4995–96. doi:10.1016/s0040-4039(01)97609-9.
- ^ Acheson, R. M.; Robinson, Robert (1952). "198. Experiments bearing on the synthesis of cortisone. Part I. Some cyclopentenone derivatives". Journal of the Chemical Society (Resumed): 1127. doi:10.1039/JR9520001127.
- ^ Ho, Tse-Lok (1992). Tandem organic reactions. New York: Wiley. ISBN 978-0-471-57022-6.
- ^ McMurry, John (2008). Organic chemistry (7th ed.). Belmont, CA: Thomson Brooks/Cole. ISBN 978-0-495-11258-7.