| |
| Identifiers | |
|---|---|
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C82H50O51 | |
| Molar mass | 1851.251 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
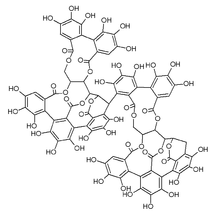
Roburin A is a tannin found in oak wood (Quercus robur and Quercus petraea[1] or Quercus alba[2]) or oak cork (Quercus suber[3]).
It is a dimeric compound, composed of two vescalagin subunits probably linked through an ether bond between the diphenoyl group (hexahydroxydiphenic acid or HHDP) of one subunit and the triphenoyl moiety (nonahydroxytriphenic acid) of the other one.[4]
- ^ Mosedale, J R; Feuillat, F; Baumes, R; Dupouey, J-L; Puech, J-L (1998). "Variability of wood extractives among Quercus roburand Quercus petraeatrees from mixed stands and their relation to wood anatomy and leaf morphology". Canadian Journal of Forest Research. 28 (7): 994–1006. doi:10.1139/x98-066.
- ^ Glabasnia, Arne; Hofmann, Thomas (2007). "Identification and Sensory Evaluation of Dehydro- and Deoxy-ellagitannins Formed upon Toasting of Oak Wood (Quercus alba L.)". Journal of Agricultural and Food Chemistry. 55 (10): 4109–18. doi:10.1021/jf070151m. PMID 17444655.
- ^ Cadahía, Estrella; Conde, Elvira; Fernández De Simón, Brígida; García-Vallejo, María Concepción (1998). "Changes in Tannic Composition of Reproduction Cork Quercus suberthroughout Industrial Processing". Journal of Agricultural and Food Chemistry. 46 (6): 2332–6. doi:10.1021/jf9709360.
- ^ Herve du Penhoat, Catherine L.M.; Michon, Veronique M.F.; Ohassan, Abdelhamid; Peng, Shuyun; Scalbert, Augustin; Gage, Douglas (1991). "Roburin A, A dimeric ellagitannin from heartwood of Quercus robur". Phytochemistry. 30 (1): 329–32. Bibcode:1991PChem..30..329H. doi:10.1016/0031-9422(91)84148-L.