 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Istodax |
| Other names | FK228; FR901228; Istodax |
| MedlinePlus | a610005 |
| License data |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Not applicable (IV only) |
| Protein binding | 92–94% |
| Metabolism | Liver (mostly CYP3A4-mediated) |
| Elimination half-life | 3 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.211.884 |
| Chemical and physical data | |
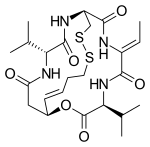
| Formula | C24H36N4O6S2 |
| Molar mass | 540.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium Chromobacterium violaceum, and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis.[2] It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene.[3]
- ^ "Romidepsin-MSN, Romidepsin-Reach (Reach Pharmaceuticals Pty Ltd)". Therapeutic Goods Administration (TGA). 13 January 2023. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ Cite error: The named reference
NCIwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Gloucesterwas invoked but never defined (see the help page).