 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Avandia |
| Other names | BRL-49653 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 99% |
| Protein binding | 99.8% |
| Metabolism | Liver (CYP2C8-mediated) |
| Elimination half-life | 3–4 hours |
| Excretion | Kidney (64%) and fecal (23%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.108.114 |
| Chemical and physical data | |
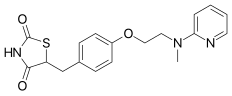
| Formula | C18H19N3O3S |
| Molar mass | 357.43 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 122 to 123 °C (252 to 253 °F) |
| |
| |
| | |
Rosiglitazone (trade name Avandia) is an antidiabetic drug in the thiazolidinedione class. It works as an insulin sensitizer, by binding to the PPAR in fat cells and making the cells more responsive to insulin. It is marketed by the pharmaceutical company GlaxoSmithKline (GSK) as a stand-alone drug or for use in combination with metformin or with glimepiride. First released in 1999, annual sales peaked at approximately $2.5-billion in 2006; however, following a meta-analysis in 2007 that linked the drug's use to an increased risk of heart attack,[1] sales plummeted to just $9.5-million in 2012. The drug's patent expired in 2012.[2]
It was patented in 1987 and approved for medical use in 1999.[3] Despite rosiglitazone's effectiveness at decreasing blood sugar in type 2 diabetes mellitus, its use decreased dramatically as studies showed apparent associations with increased risks of heart attacks and death.[4] Adverse effects alleged to be caused by rosiglitazone were the subject of over 13,000 lawsuits against GSK;[5] as of July 2010, GSK had agreed to settlements on more than 11,500 of these suits.
Some reviewers recommended rosiglitazone be taken off the market, but an FDA panel disagreed, and it remains available in the U.S.[6] From November 2011 until November 2013, the federal government did not allow Avandia to be sold without a prescription from a certified doctor; moreover, patients were required to be informed of the risks associated with its use, and the drug had to be purchased by mail order through specified pharmacies.[7] In 2013, the FDA lifted its earlier restrictions on rosiglitazone after reviewing the results of a 2009 trial which failed to show increased heart attack risk.[8][9]
In Europe, the European Medicines Agency (EMA) recommended in September 2010 that the drug be suspended because the benefits no longer outweighed the risks.[10][11] It was withdrawn from the market in the UK, Spain and India in 2010,[12] and in New Zealand and South Africa in 2011.[13]
- ^ Nissen SE, Wolski K (June 2007). "Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes". The New England Journal of Medicine. 356 (24): 2457–2471. doi:10.1056/NEJMoa072761. PMID 17517853. S2CID 46431986.
- ^ US 5002953, Hindley RM, "Novel compounds", published 1991-03-26, assigned to Beecham Group plc
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 450. ISBN 9783527607495.
- ^ Chen X, Yang L, Zhai SD (December 2012). "Risk of cardiovascular disease and all-cause mortality among diabetic patients prescribed rosiglitazone or pioglitazone: a meta-analysis of retrospective cohort studies". Chinese Medical Journal. 125 (23): 4301–4306. PMID 23217404.
- ^ "Glaxo Withheld Avandia Study, Ex-Regulator Said to Testify". Bloomberg.
- ^ Harris G (February 19, 2010). "Controversial Diabetes Drug Harms Heart, U.S. Concludes". New York Times.
- ^ "Most Popular E-mail Newsletter". USA Today. 2011-05-24. Archived from the original on 2018-07-20. Retrieved 2011-05-19.
- ^ "Glaxo's Avandia Cleared From Sales Restrictions by FDA". Bloomberg.
- ^ U.S. Food and Drug Administration (November 25, 2013). "FDA requires removal of certain restrictions on the diabetes drug Avandia".
- ^ "European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim". News and Events. European Medicines Agency. 2018-09-17. Archived from the original on 2015-09-24. Retrieved 2010-10-31.
- ^ "Call to 'suspend' diabetes drug". BBC News. 2010-09-23.
- ^ "Drugs banned in India". Central Drugs Standard Control Organization, Dte.GHS, Ministry of Health and Family Welfare, Government of India. Archived from the original on 2015-02-21. Retrieved 2013-09-17.
- ^ "Diabetes drug withdrawn". Stuff.co.nz. NZPA. 17 February 2011. Archived from the original on 13 October 2013. Retrieved 5 November 2011.