| |

| |

| |
| Names | |
|---|---|
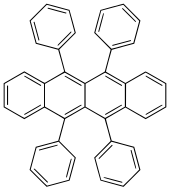
| Preferred IUPAC name
5,6,11,12-Tetraphenyltetracene | |
| Other names
5,6,11,12-Tetraphenylnaphthacene, rubrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.494 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C42H28 | |
| Molar mass | 532.7 g/mol |
| Melting point | 315 °C (599 °F; 588 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rubrene (5,6,11,12-tetraphenyltetracene) is the organic compound with the formula (C18H8(C6H5)4. It is a red colored polycyclic aromatic hydrocarbon. Because of its distinctive optical and electrical properties, rubrene has been extensively studied. It has been used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks.[1]
- ^ Sawatzki-Park, Michael; Wang, Shu-Jen; Kleemann, Hans; Leo, Karl (2023). "Highly Ordered Small Molecule Organic Semiconductor Thin-Films Enabling Complex, High-Performance Multi-Junction Devices". Chemical Reviews. 123 (13): 8232–8250. doi:10.1021/acs.chemrev.2c00844. PMC 10347425. PMID 37315945.