 | |
| Clinical data | |
|---|---|
| Trade names | Banzel, Inovelon |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609001 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 85% (under fed conditions); tmax = 4–6 hours |
| Protein binding | 34% |
| Metabolism | Carboxylesterase-mediated hydrolysis (CYP not involved) |
| Metabolites | Inactive |
| Elimination half-life | 6–10 hours |
| Excretion | Urine (85%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
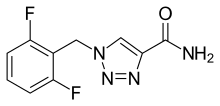
| Formula | C10H8F2N4O |
| Molar mass | 238.198 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rufinamide is an anticonvulsant medication. It is used in combination with other medication and therapy to treat Lennox–Gastaut syndrome[3] and various other seizure disorders. Rufinamide, a triazole derivative, was developed in 2004 by Novartis Pharma, AG, and is manufactured by Eisai.
Rufinamide was approved by the US Food and Drug Administration (FDA) in November 2008, as adjunctive treatment of seizures associated with Lennox-Gastaut syndrome in children four years and older and adults. Its official FDA-approved labeling does not mention use in the treatment of partial seizures inasmuch as clinical trials submitted to the FDA were marginal. However, several recent clinical trials suggest that the drug has efficacy for partial seizures [4] It is marketed under the brand name Banzel.[5] It is also marketed in the European Union under the brand name Inovelon.[6] It is available as a generic medication.[7]
The mechanism of action of rufinamide is not fully understood. There is some evidence that rufinamide can modulate the gating of voltage-gated sodium channels,[8][9] a common target for antiepileptic drugs.[10] A recent study indicates subtle effects on the voltage-dependence of gating and the time course of inactivation in some sodium channel isoforms that could reduce neuronal excitability.[11] However, this action cannot explain the unique spectrum of activity of rufinamide.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ a b "Banzel- rufinamide tablet, film coated Banzel- rufinamide suspension". DailyMed. 15 April 2020. Retrieved 21 October 2020.
- ^ Hakimian S, Cheng-Hakimian A, Anderson GD, Miller JW (August 2007). "Rufinamide: a new anti-epileptic medication". Expert Opinion on Pharmacotherapy. 8 (12): 1931–1940. doi:10.1517/14656566.8.12.1931. PMID 17696794. S2CID 19522242.
- ^ Brodie MJ, Rosenfeld WE, Vazquez B, Sachdeo R, Perdomo C, Mann A, Arroyo S (August 2009). "Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo-controlled trial". Epilepsia. 50 (8): 1899–1909. doi:10.1111/j.1528-1167.2009.02160.x. PMID 19490053. S2CID 38485532.
- ^ FDA press release - FDA Approves New Drug to Treat Severe Form of Epilepsy
- ^ "European Public Assessment Report for rufinamide (INOVELON)". Archived from the original on 14 March 2009. Retrieved 25 November 2008.
- ^ "2022 First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). 3 March 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
- ^ Rogawski MA (June 2006). "Diverse mechanisms of antiepileptic drugs in the development pipeline". Epilepsy Research. 69 (3): 273–294. doi:10.1016/j.eplepsyres.2006.02.004. PMC 1562526. PMID 16621450.
- ^ Striano P, McMurray R, Santamarina E, Falip M (February 2018). "Rufinamide for the treatment of Lennox-Gastaut syndrome: evidence from clinical trials and clinical practice". Epileptic Disorders. 20 (1): 13–29. doi:10.1684/epd.2017.0950. PMID 29313492.
- ^ Rogawski MA, Löscher W (July 2004). "The neurobiology of antiepileptic drugs". Nature Reviews. Neuroscience. 5 (7): 553–564. doi:10.1038/nrn1430. PMID 15208697. S2CID 2201038.
- ^ Gilchrist J, Dutton S, Diaz-Bustamante M, McPherson A, Olivares N, Kalia J, et al. (May 2014). "Nav1.1 modulation by a novel triazole compound attenuates epileptic seizures in rodents". ACS Chemical Biology. 9 (5): 1204–1212. doi:10.1021/cb500108p. PMC 4027953. PMID 24635129.