| |

| |

| |
| Names | |
|---|---|
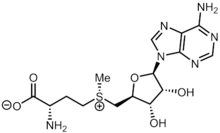
| Systematic IUPAC name
(2S)-2-Amino-4-[(S)-{[(2S,3S,4R,5R)-5-(4-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}methylsulfaniumyl]butanoate | |
| Other names
S-Adenosyl-L-methionine; SAM-e; SAMe, AdoMet, Heparab (India), ademethionine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.045.391 |
| KEGG | |
| MeSH | S-Adenosylmethionine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H22N6O5S | |
| Molar mass | 398.44 g·mol−1 |
| Pharmacology | |
| A16AA02 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver.[1] More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952.[1]
In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response;[2] amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and signaling molecule.[3]
- ^ a b Cantoni, GL (1952). "The Nature of the Active Methyl Donor Formed Enzymatically from L-Methionine and Adenosinetriphosphate". J Am Chem Soc. 74 (11): 2942–3. doi:10.1021/ja01131a519.
- ^ Ding, Wei; Smulan, Lorissa J.; Hou, Nicole S.; Taubert, Stefan; Watts, Jennifer L.; Walker, Amy K. (2015-10-06). "S-Adenosylmethionine Levels Govern Innate Immunity through Distinct Methylation-Dependent Pathways". Cell Metabolism. 22 (4): 633–645. doi:10.1016/j.cmet.2015.07.013. PMC 4598287. PMID 26321661.
- ^ Wang, X.; Oh, M. W.; Komatsu, S. (2016-06-01). "Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses". Biologia Plantarum. 60 (2): 269–278. doi:10.1007/s10535-016-0586-6. ISSN 0006-3134. S2CID 15567646.