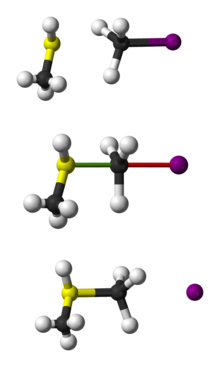
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted (i.e. simultaneous) fashion.
The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in SN1.
The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry.