The ST motif is a commonly occurring feature in proteins and polypeptides. It consists of four or five amino acid residues with either serine or threonine as the first residue (residue i).[1][2][3] It is defined by two internal hydrogen bonds. One is between the side chain oxygen of residue i and the main chain NH of residue i + 2 or i + 3; the other is between the main chain oxygen of residue i and the main chain NH of residue i + 3 or i + 4. Two websites are available for finding and examining ST motifs in proteins, Motivated Proteins:[4][5] and PDBeMotif.[6] [7]
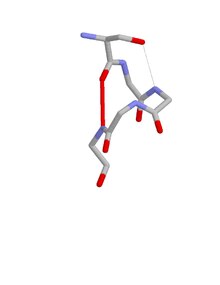
When one of the hydrogen bonds is between the main chain oxygen of residue i and the side chain NH of residue i + 3 the motif incorporates a beta turn. When one of the hydrogen bonds is between the side chain oxygen of residue i and the main chain NH of residue i + 2 the motif incorporates an ST turn.
As with ST turns, a significant proportion of ST motifs occur at the N-terminus of an alpha helix with the serine or threonine as the N cap residue. They have thus often been described as helix capping features.[8][9][10][11]
A related motif is the asx motif which has aspartate or asparagine as the first residue.
Two well conserved threonines at α-helical N-termini occur as ST motifs and form part of the characteristic nucleotide binding sites of SF1 and SF2 type DNA and RNA helicases.[12]
It has been suggested that the sequences SPXX or STXX are frequently found at DNA-binding sites and also that they are recognized as substrates by some protein kinases. Structural studies of polypeptides indicate that such tetrapeptides can adopt the hydrogen bonding pattern of the ST motif.[13][14]
- ^ Wan, WY; Milner-White EJ (1999). "A recurring two-hydrogen bond motif incorporating a serine or threonine residue is found both at alpha-helical N-termini and in other situations". Journal of Molecular Biology. 286 (5): 1650–1666. doi:10.1006/jmbi.1999.2551. PMID 10064721.
- ^ Atkinson, A; Graton J (2014). "Insights into a highly conserved network of hydrogen bonds in the agonist binding site of nicotinic acetylcholine receptors". Proteins. 82 (10): 2303–2317. doi:10.1002/prot.24589. PMID 24752960.
- ^ Quan, A; Robinson PJ (2013). "Syndapin - A membrane remodelling and endocytotic F-BAR protein". FEBS Journal. 280 (21): 5198–5212. doi:10.1111/febs.12343. PMID 23668323.
- ^ "Small Three-Dimensional Protein Motifs".
- ^ Leader, DP; Milner-White EJ (2009). "Motivated Proteins: A web application for studying small three-dimensional protein motifs". BMC Bioinformatics. 10 (1): 60. doi:10.1186/1471-2105-10-60. PMC 2651126. PMID 19210785.
- ^ "PDBeMotif".
- ^ Golovin, A; Henrick K (2008). "MSDmotif: exploring protein sites and motifs". BMC Bioinformatics. 9 (1): 312. doi:10.1186/1471-2105-9-312. PMC 2491636. PMID 18637174.
- ^ Presta, LG; Rose GD (1988). "Helix Caps". Science. 240 (4859): 1632–1641. doi:10.1126/science.2837824. PMID 2837824.
- ^ Doig, AJ; MacArthur MW (1997). "Structures of N-termini of helices in proteins". Protein Science. 6 (1): 147–155. doi:10.1002/pro.5560060117. PMC 2143508. PMID 9007987.
- ^ Aurora, R; Rose GD (1998). "Helix Capping". Protein Science. 7 (1): 21–38. doi:10.1002/pro.5560070103. PMC 2143812. PMID 9514257.
- ^ Gunasekaran, K; Nagarajam HA (1998). "Stereochemical punctuation marks in protein structure". Journal of Molecular Biology. 275 (5): 917–932. doi:10.1006/jmbi.1997.1505. PMID 9480777. S2CID 35919397.
- ^ Milner-White, EJ; Pietras Z; Luisi BF (2010). "An ancient anion-binding structural module in RNA and DNA helicases". Proteins. 78 (8): 1900–1908. doi:10.1002/prot.22704. PMC 7610952. PMID 20310069.
- ^ Suzuki, M (1989). "SPXX, a frequent sequence motif in gene regulatory proteins". Journal of Molecular Biology. 207 (1): 61–84. doi:10.1016/0022-2836(89)90441-5. PMID 2500531.
- ^ Song, B; Bomar MG; Kibler P; Kodukula K; Galande AK (2012). "The Serine-Proline Turn:A novel hydrogen-bonded template for designing peptidomimetics". Organic Letters. 14 (3): 732–735. doi:10.1021/ol203272k. PMID 22257322.