 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Invirase, Fortovase |
| Other names | SQV |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696001 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~4% (without ritonavir boosting)[3] |
| Protein binding | 98% |
| Metabolism | Liver, mainly by CYP3A4 |
| Elimination half-life | 9–15 hours |
| Excretion | feces (81%) and urine (3%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
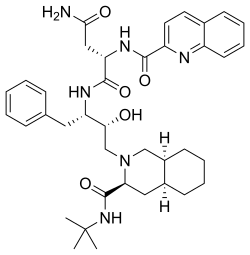
| Formula | C38H50N6O5 |
| Molar mass | 670.855 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Saquinavir, sold under the brand name Invirase among others, is an antiretroviral medication used together with other medications to treat or prevent HIV/AIDS.[4] Typically it is used with ritonavir or lopinavir/ritonavir to increase its effect.[4] It is taken by mouth.[4]
Common side effects include nausea, vomiting, diarrhea, and feeling tired.[4] More serious side effects include problems with QT prolongation, heart block, high blood lipids, and liver problems.[4] It appears to be safe in pregnancy.[4] It is in the protease inhibitor class and works by blocking the HIV protease.[4]
Saquinavir was patented in 1988 and first sold in 1995.[5][6]
- ^ "Saquinavir Use During Pregnancy". Drugs.com. 20 March 2018. Retrieved 28 January 2020.
- ^ Roche Products Pty Limited (6 November 2018). "Invirase® (Saquinavir mesilate)". Australian Product Information – via MedAdvisor International Pty Ltd.
- ^ "Invirase- saquinavir mesylate capsule INVIRASE- saquinavir mesylate tablet, film coated". DailyMed. 26 December 2019. Retrieved 28 January 2020.
- ^ a b c d e f g "Saquinavir". The American Society of Health-System Pharmacists. Archived from the original on 8 September 2015. Retrieved 5 September 2015.
- ^ Minor LK (2006). Handbook of Assay Development in Drug Discovery. Hoboken: CRC Press. p. 117. ISBN 9781420015706. Archived from the original on 31 March 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 509. ISBN 9783527607495.