 | |
| Clinical data | |
|---|---|
| Pronunciation | sar"e sye' kleen |
| Trade names | Seysara |
| Other names | P-005672 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618068 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.241.852 |
| Chemical and physical data | |
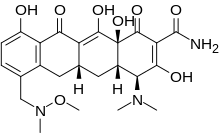
| Formula | C24H29N3O8 |
| Molar mass | 487.509 g·mol−1 |
| 3D model (JSmol) | |
| |
Sarecycline, sold under the brand name Seysara, is a narrow-spectrum tetracycline-derived antibiotic medication.[2][3] It is specifically designed for the treatment of acne, and was approved by the FDA in October 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older.[1] Two randomized and well-controlled clinical trials reported efficacy data on both facial and truncal acne (back and chest).[4] Efficacy was assessed in a total of 2002 subjects 9 years of age and older.[1] Unlike other tetracycline-class antibiotics, sarecycline has a long C7 moiety that extends into and directly interact with the bacterial messenger RNA (mRNA).[5] The spectrum of activity is limited to clinically relevant Gram-positive bacteria, mainly Cutibacterium acnes, with little or no activity against Gram-negative bacterial microflora commonly found in the human gastrointestinal tract.[6]
- ^ a b c "Seysara- sarecycline hydrochloride tablet, coated". DailyMed. U.S. National Library of Medicine. 1 August 2021. Retrieved 31 March 2023.
- ^ Zhanel G, Critchley I, Lin LY, Alvandi N (January 2019). "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy. 63 (1). doi:10.1128/AAC.01297-18. PMC 6325184. PMID 30397052.
- ^ "Sarecycline". PubChem. U.S. National Library of Medicine. Retrieved 5 September 2020.
- ^ Moore A, Green LJ, Bruce S, Sadick N, Tschen E, Werschler P, et al. (September 2018). "Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials". Journal of Drugs in Dermatology. 17 (9): 987–996. PMID 30235387.
- ^ Batool Z, Lomakin IB, Polikanov YS, Bunick CG (August 2020). "Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome". Proceedings of the National Academy of Sciences of the United States of America. 117 (34): 20530–20537. Bibcode:2020PNAS..11720530B. doi:10.1073/pnas.2008671117. PMC 7456112. PMID 32817463.
- ^ Zhanel G, Critchley I, Lin LY, Alvandi N (January 2019). "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy. 63 (1). doi:10.1128/AAC.01297-18. PMC 6325184. PMID 30397052.