 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /səˈlɛdʒɪliːn/ sə-LEJ-i-leen ("seh-LEH-ji-leen")[1][2] |
| Trade names | Eldepryl, Jumex, Zelapar, Emsam, Anipryl, others[3] |
| Other names | L-Deprenyl; L-Deprenil; L-Deprenalin; L-Deprenaline; L-E-250; l-E-250; L-Phenylisopropyl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth,[4][5] buccal (ODT),[6][7] transdermal[8][9] |
| Drug class | MAO-B inhibitor; Norepinephrine releasing agent; Antidepressant |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 4–10%[5][11][12] ODT: ~5–8× oral[13][7][14] Transdermal: 75%[9] |
| Protein binding | 85–90%[9][8][6] |
| Metabolism | Liver, other tissues (CYP2B6, CYP2C19, others)[5][21][9][22] |
| Metabolites | • Desmethylselegiline (DMS) • Levomethamphetamine • Levoamphetamine |
| Onset of action | Oral: ≤1 hour[15][16] |
| Elimination half-life | Oral: • S (single): 1.2–3.5 h[5] • S (multi): 7.7–9.7 h[5][12] • DMS (single): 2.2–3.8 h[5] • DMS (multi): 9.5 h[5] • L-MA: 14–21 h[5][7] • L-A: 16–18 h[5][7] ODT: • S (single): 1.3 h[6] • S (multi): 10 h[6] Patch: • S: 20 h[12][8] |
| Duration of action | |
| Excretion | Urine (87%):[18][19][7][5][20] • L-MA: 20–63% • L-A: 9–26% • DMS: 1% • S: 0.01–0.03% Feces: 15%[18][7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.109.269 |
| Chemical and physical data | |
| Formula | C13H17N |
| Molar mass | 187.286 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Levorotatory enantiomer |
| |
| |
| (verify) | |
Selegiline, also known as L-deprenyl and sold under the brand names Eldepryl, Zelapar, and Emsam among others, is a medication which is used in the treatment of Parkinson's disease and major depressive disorder.[4][6][8][3] It has also been studied and used off-label for a variety of other indications, but has not been formally approved for any other use.[23][24] The medication, in the form licensed for depression, has modest effectiveness for this condition that is similar to that of other antidepressants.[24][25][26] Selegiline is provided as a swallowed tablet or capsule[4][5] or an orally disintegrating tablet (ODT)[6][7] for Parkinson's disease and as a patch applied to skin for depression.[8][9]
Side effects of selegiline occurring more often than with placebo include insomnia, dry mouth, dizziness, anxiety, abnormal dreams, and application site reactions (with the patch form), among others.[24][25][27][4][8] At high doses, selegiline has the potential for dangerous food and drug interactions, such as tyramine-related hypertensive crisis (the so-called "cheese reaction") and risk of serotonin syndrome.[9][28][5] However, doses within the approved clinical range appear to have little to no risk of these interactions.[9][28][5] In addition, the ODT and transdermal patch forms of selegiline have reduced risks of such interactions compared to the conventional oral form.[7][9] Selegiline has no known misuse potential or dependence liability and is not a controlled substance except in Japan.[29][30][31][32][8][33]
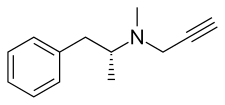
Selegiline acts as a monoamine oxidase inhibitor (MAOI) and thereby increases levels of monoamine neurotransmitters in the brain.[17][11][28][5] At typical clinical doses used for Parkinson's disease, selegiline is a selective and irreversible inhibitor of monoamine oxidase B (MAO-B), increasing brain levels of dopamine.[17][11][28][5] At higher doses, it loses its specificity for MAO-B and also inhibits monoamine oxidase A (MAO-A), which increases serotonin and norepinephrine levels in the brain as well.[17][11][28][5] In addition to its MAOI activity, selegiline is a catecholaminergic activity enhancer (CAE) and enhances the impulse-mediated release of norepinephrine and dopamine in the brain.[34][35][36][37][28] This action may be mediated by TAAR1 agonism.[38][39][40] After administration, selegiline partially metabolizes into levomethamphetamine and levoamphetamine, which act as norepinephrine releasing agents (NRAs) and may contribute to its therapeutic and adverse effects as well.[41][31][42] The levels of these metabolites are much lower with the ODT and transdermal patch forms of selegiline.[7][9] Chemically, selegiline is a substituted phenethylamine and amphetamine,[43] a derivative of methamphetamine,[43] and the purified levorotatory enantiomer of deprenyl (the racemic mixture of selegiline and D-deprenyl).[44][23]
Deprenyl was discovered and studied as an antidepressant in the early 1960s by Zoltan Ecseri, József Knoll, and other colleagues at Chinoin Pharmaceutical Company in Hungary.[44][23] Subsequently, selegiline was purified from deprenyl and was studied and developed itself.[44] Selegiline was first introduced for medical use, to treat Parkinson's disease, in Hungary in 1977.[45] It was subsequently approved in the United Kingdom in 1982 and in the United States in 1989.[45][46] The ODT was approved for Parkinson's disease in the United States in 2006 and in the European Union in 2010, while the patch was introduced for depression in the United States in 2006.[45][23] Selegiline was the first selective MAO-B inhibitor to be discovered and marketed.[13][47][48] In addition to its medical use, there has been interest in selegiline as a potential anti-aging drug and nootropic.[49][50][51] However, effects of this sort are controversial and uncertain.[49][52][53][54] Generic versions of selegiline are available in the case of the conventional oral form, but not in the case of the ODT or transdermal patch forms.[55][56]
- ^ Cite error: The named reference
Parkinsons.org2018was invoked but never defined (see the help page). - ^ Cite error: The named reference
Acosta2020was invoked but never defined (see the help page). - ^ a b "Selegiline". Drugs.com. Archived from the original on July 3, 2024. Retrieved February 7, 2016.
- ^ a b c d Cite error: The named reference
PillLabelwas invoked but never defined (see the help page). - ^ a b c d e f g h i j k l m n o p Mahmood I (August 1997). "Clinical pharmacokinetics and pharmacodynamics of selegiline. An update". Clin Pharmacokinet. 33 (2): 91–102. doi:10.2165/00003088-199733020-00002. PMID 9260033.
- ^ a b c d e f Cite error: The named reference
ODTLabelwas invoked but never defined (see the help page). - ^ a b c d e f g h i Poston KL, Waters C (October 2007). "Zydis selegiline in the management of Parkinson's disease". Expert Opin Pharmacother. 8 (15): 2615–2624. doi:10.1517/14656566.8.15.2615. PMID 17931095.
- ^ a b c d e f g "EMSAM® (Selegiline Transdermal System) Label" (PDF). Food and Drug Administration. July 2017. Retrieved July 2, 2024.
- ^ a b c d e f g h i Cite error: The named reference
LeeChen2007was invoked but never defined (see the help page). - ^ Anvisa (March 31, 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published April 4, 2023). Archived from the original on August 3, 2023. Retrieved August 16, 2023.
- ^ a b c d Magyar K (2011). "The Pharmacology of Selegiline". In Youdim M, Riederer P (eds.). Monoamine Oxidases and Their Inhibitors. International Review of Neurobiology. Vol. 100. Academic Press. pp. 65–84. doi:10.1016/B978-0-12-386467-3.00004-2. ISBN 978-0-12-386467-3. PMID 21971003.
- ^ a b c Pae CU, Lim HK, Han C, Neena A, Lee C, Patkar AA (August 2007). "Selegiline transdermal system: current awareness and promise". Prog Neuropsychopharmacol Biol Psychiatry. 31 (6): 1153–1163. doi:10.1016/j.pnpbp.2007.04.020. PMID 17614182.
- ^ a b Löhle M, Storch A (November 2008). "Orally disintegrating selegiline for the treatment of Parkinson's disease". Expert Opin Pharmacother. 9 (16): 2881–2891. doi:10.1517/14656566.9.16.2881. PMID 18937619.
- ^ Clarke A, Brewer F, Johnson ES, Mallard N, Hartig F, Taylor S, et al. (November 2003). "A new formulation of selegiline: improved bioavailability and selectivity for MAO-B inhibition". Journal of Neural Transmission. 110 (11): 1241–1255. doi:10.1007/s00702-003-0036-4. PMID 14628189. S2CID 711419.
- ^ a b Cite error: The named reference
Knoll1983was invoked but never defined (see the help page). - ^ Knoll J (1986). "Role of B-Type Monoamine Oxidase Inhibition in the Treatment of Parkinson's Disease". Movement Disorders. Boston, MA: Springer US. p. 53–81. doi:10.1007/978-1-4684-5038-5_3. ISBN 978-1-4684-5040-8.
- ^ a b c d Heinonen EH, Lammintausta R (1991). "A review of the pharmacology of selegiline". Acta Neurologica Scandinavica. Supplementum. 136: 44–59. doi:10.1111/j.1600-0404.1991.tb05020.x. PMID 1686954.
- ^ a b Heinonen EH, Anttila MI, Lammintausta RA (December 1994). "Pharmacokinetic aspects of l-deprenyl (selegiline) and its metabolites". Clin Pharmacol Ther. 56 (6 Pt 2): 742–749. doi:10.1038/clpt.1994.204. PMID 7995016.
- ^ Heinonen EH, Myllylä V, Sotaniemi K, Lamintausta R, Salonen JS, Anttila M, et al. (November 1989). "Pharmacokinetics and metabolism of selegiline". Acta Neurologica Scandinavica. Supplementum. 126: 93–99. doi:10.1111/j.1600-0404.1989.tb01788.x. PMID 2515726. S2CID 221440315.
- ^ Chrisp P, Mammen GJ, Sorkin EM (May 1991). "Selegiline: A Review of its Pharmacology, Symptomatic Benefits and Protective Potential in Parkinson's Disease". Drugs Aging. 1 (3): 228–248. doi:10.2165/00002512-199101030-00006. PMID 1794016.
- ^ Rodrigues AD (June 2022). "Drug Interactions Involving 17α-Ethinylestradiol: Considerations Beyond Cytochrome P450 3A Induction and Inhibition". Clin Pharmacol Ther. 111 (6): 1212–1221. doi:10.1002/cpt.2383. PMID 34342002.
- ^ Hidestrand M, Oscarson M, Salonen JS, Nyman L, Pelkonen O, Turpeinen M, et al. (November 2001). "CYP2B6 and CYP2C19 as the major enzymes responsible for the metabolism of selegiline, a drug used in the treatment of Parkinson's disease, as revealed from experiments with recombinant enzymes". Drug Metab Dispos. 29 (11): 1480–1484. PMID 11602525.
- ^ a b c d Cite error: The named reference
Miklya2016was invoked but never defined (see the help page). - ^ a b c Cite error: The named reference
RossanoCaiazzaSobrino2023was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
CitromeGoldbergPortland2013was invoked but never defined (see the help page). - ^ Cite error: The named reference
CiprianiFurukawaSalanti2018was invoked but never defined (see the help page). - ^ Cite error: The named reference
RobinsonAmsterdam2008was invoked but never defined (see the help page). - ^ a b c d e f Gerlach M, Youdim MB, Riederer P (December 1996). "Pharmacology of selegiline". Neurology. 47 (6 Suppl 3): S137–S145. doi:10.1212/wnl.47.6_suppl_3.137s. PMID 8959982.
- ^ Cite error: The named reference
FinbergRabey2016was invoked but never defined (see the help page). - ^ Cite error: The named reference
FabbriniAbbruzzeseMarconi2012was invoked but never defined (see the help page). - ^ a b Yasar S, Goldberg JP, Goldberg SR (January 1, 1996). "Are metabolites of l-deprenyl (Selegiline) useful or harmful? Indications from preclinical research". Deprenyl — Past and Future. Journal of Neural Transmission. Supplementum. Vol. 48. pp. 61–73. doi:10.1007/978-3-7091-7494-4_6. ISBN 978-3-211-82891-5. PMID 8988462.
- ^ Cite error: The named reference
NickelSzelenyiSchulze1994was invoked but never defined (see the help page). - ^ Cite error: The named reference
KEGGwas invoked but never defined (see the help page). - ^ Knoll J (1997). "Istoriia deprenil--pervogo selektivnogo ingibitora monoaminoksidazy tipa B" [History of deprenyl--the first selective inhibitor of monoamine oxidase type B]. Voprosy Meditsinskoi Khimii. 43 (6): 482–493. PMID 9503565.
- ^ Knoll J (February 1998). "(-)Deprenyl (selegiline), a catecholaminergic activity enhancer (CAE) substance acting in the brain". Pharmacol Toxicol. 82 (2): 57–66. doi:10.1111/j.1600-0773.1998.tb01399.x. PMID 9498233.
- ^ Cite error: The named reference
Miklya2014awas invoked but never defined (see the help page). - ^ Cite error: The named reference
GasznerMiklya2006was invoked but never defined (see the help page). - ^ Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ Shimazu S, Miklya I (May 2004). "Pharmacological studies with endogenous enhancer substances: β-phenylethylamine, tryptamine, and their synthetic derivatives". Prog Neuropsychopharmacol Biol Psychiatry. 28 (3): 421–427. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948.
- ^ Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Rev Recent Clin Trials. 2 (1): 3–19. doi:10.2174/157488707779318107. PMID 18473983.
- ^ Gerlach M, Reichmann H, Riederer P (2012). "A critical review of evidence for preclinical differences between rasagiline and selegiline". Basal Ganglia. 2 (4): S9–S15. doi:10.1016/j.baga.2012.04.032.
- ^ Rothman RB, Baumann MH (October 2003). "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol. 479 (1–3): 23–40. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ^ a b Kraemer T, Maurer HH (April 2002). "Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives". Ther Drug Monit. 24 (2): 277–289. doi:10.1097/00007691-200204000-00009. PMID 11897973.
- ^ a b c Parnham MJ (1993). "The History of l-Deprenyl". Inhibitors of Monoamine Oxidase B: Pharmacology and Clinical Use in Neurodegenerative Disorders. Milestones in Drug Therapy. Basel: Birkhäuser Basel. pp. 237–251. doi:10.1007/978-3-0348-6348-3_12. ISBN 978-3-0348-6349-0.
- ^ a b c Tábi T, Vécsei L, Youdim MB, Riederer P, Szökő É (May 2020). "Selegiline: a molecule with innovative potential". J Neural Transm (Vienna). 127 (5): 831–842. doi:10.1007/s00702-019-02082-0. PMC 7242272. PMID 31562557.
- ^ Cite error: The named reference
MylanHist2011was invoked but never defined (see the help page). - ^ Cite error: The named reference
HoffmanOlsonSchoffstall2023was invoked but never defined (see the help page). - ^ Cite error: The named reference
Golbe1988was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
SchifanoCatalaniSharif2022was invoked but never defined (see the help page). - ^ Knoll J (2001). "Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain". CNS Drug Rev. 7 (3): 317–345. doi:10.1111/j.1527-3458.2001.tb00202.x. PMC 6494119. PMID 11607046.
- ^ Schneider LS, Tariot PN, Goldstein B (December 1994). "Therapy with l-deprenyl (selegiline) and relation to abuse liability". Clin Pharmacol Ther. 56 (6 Pt 2): 750–756. doi:10.1038/clpt.1994.205. PMID 7995017.
- ^ Blazer DG, Yaffe K, Liverman CT (July 21, 2015). Risk and Protective Factors and Interventions: General Cognitive Aging Interventions and Next Steps. National Academies Press (US). Retrieved July 5, 2024.
- ^ Brown RP, Gerbarg PL (2008). Muskin PR (ed.). "Integrative Psychopharmacology: A Practical Approach to Herbs and Nutrients in Psychiatry". Review of Psychiatry. Complementary and Alternative Medicine and Psychiatry. 19 (1). American Psychiatric Publishing: 1–66 (39). ISBN 978-1-58562-827-8. Retrieved July 5, 2024.
- ^ Finberg JP (April 2019). "Inhibitors of MAO-B and COMT: their effects on brain dopamine levels and uses in Parkinson's disease". Journal of Neural Transmission. 126 (4): 433–448. doi:10.1007/s00702-018-1952-7. PMID 30386930.
- ^ Cite error: The named reference
Drugs@FDAwas invoked but never defined (see the help page). - ^ Cite error: The named reference
AsnisHenderson2014was invoked but never defined (see the help page).