| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
| ||
| ChemSpider | |||
| ECHA InfoCard | 100.033.972 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
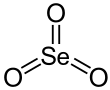
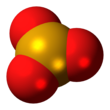
| SeO3 | |||
| Molar mass | 126.96 g/mol | ||
| Appearance | white hygroscopic crystals | ||
| Density | 3.44 g/cm3 | ||
| Melting point | 118.35 °C (245.03 °F; 391.50 K) | ||
| Boiling point | sublimes | ||
| very soluble | |||
| Structure | |||
| tetragonal | |||
| Hazards | |||
| GHS labelling:[3] | |||
 
| |||
| Danger | |||
| H301, H331, H373, H410 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
7 mg/kg (rat, oral) 7.08 mg/kg (mouse, oral) 5.06 mg/kg (guinea pig, oral) 2.25 mg/kg (rabbit, oral) 13 mg/kg (horse, oral)[2] | ||
LC50 (median concentration)
|
13 mg/kg (pig, oral) 9.9 mg/kg (cow, oral) 3.3 mg/kg (goat, oral) 3.3 mg/kg (sheep, oral)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.[4]
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–81. ISBN 0-8493-0594-2.
- ^ a b "Selenium compounds (as Se)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "C&L Inventory". echa.europa.eu. Retrieved 16 December 2021.
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515