| |
| |
| Names | |
|---|---|
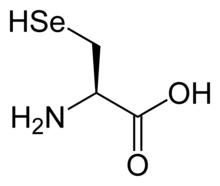
| IUPAC name
Selenocysteine
| |
| Systematic IUPAC name
3-Selanyl-L-alanine (semisystematic name)
2-Amino-3-selanylpropanoic acid (fully systematic name) | |
| Other names
L-Selenocysteine; Selenium-cysteine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.236.386 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H7NO2Se | |
| Molar mass | 168.065 g·mol−1 |
| Properties | |
| Acidity (pKa) | 5.24,[2] 5.43[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Selenocysteine (symbol Sec or U,[4] in older publications also as Se-Cys)[5] is the 21st proteinogenic amino acid. Selenoproteins contain selenocysteine residues. Selenocysteine is an analogue of the more common cysteine with selenium in place of the sulfur.
Selenocysteine is present in several enzymes (for example glutathione peroxidases, tetraiodothyronine 5′ deiodinases, thioredoxin reductases, formate dehydrogenases, glycine reductases, selenophosphate synthetase 2, methionine-R-sulfoxide reductase B1 (SEPX1), and some hydrogenases). It occurs in all three domains of life, including important enzymes (listed above) present in humans.[6]
Selenocysteine was discovered in 1974[7] by biochemist Thressa Stadtman at the National Institutes of Health.[8]
- ^ Merck Index, 12th Edition, 8584
- ^ Huber RE, Criddle RS (1967-10-01). "Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs". Archives of Biochemistry and Biophysics. 122 (1): 164–173. doi:10.1016/0003-9861(67)90136-1. ISSN 0003-9861. PMID 6076213.
- ^ Thapa B, Schlegel HB (2016-11-10). "Theoretical Calculation of p K a 's of Selenols in Aqueous Solution Using an Implicit Solvation Model and Explicit Water Molecules". The Journal of Physical Chemistry A. 120 (44): 8916–8922. Bibcode:2016JPCA..120.8916T. doi:10.1021/acs.jpca.6b09520. ISSN 1089-5639. PMID 27748600.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ "IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN) and Nomenclature Committee of IUBMB (NC-IUBMB)". European Journal of Biochemistry. 264 (2): 607–609. 17 August 1999. doi:10.1046/j.1432-1327.1999.news99.x.
- ^ Johansson L, Gafvelin G, Arnér ES (October 2005). "Selenocysteine in proteins—properties and biotechnological use". Biochimica et Biophysica Acta (BBA) - General Subjects. 1726 (1): 1–13. doi:10.1016/j.bbagen.2005.05.010. hdl:10616/39311. PMID 15967579.
- ^ "Stadtman Pioneer of Selenium Biochemistry - Office of NIH History and Stetten Museum". history.nih.gov. Retrieved 2023-04-06.
- ^ Stadtman TC (March 1974). "Selenium biochemistry". Science. 183 (4128): 915–22. Bibcode:1974Sci...183..915S. doi:10.1126/science.183.4128.915. PMID 4605100. S2CID 84982102.