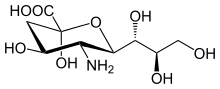
 N-Acetylneuraminic acid, the most common of the sialic acids |
Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone.[1] The term "sialic acid" (from Greek σίαλον (síalon) 'saliva') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is N-acetylneuraminic acid (Neu5Ac or NANA) found in animals and some prokaryotes.
Sialic acids are found widely distributed in animal tissues and related forms are found to a lesser extent in other organisms like in some micro-algae,[2] bacteria and archaea.[3][4][5][6] Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins.[7] However, sialic acids have been also observed in Drosophila embryos and other insects.[8] Generally, plants seem not to contain or display sialic acids.[9]
In humans, the brain has the highest sialic acid content, where these acids play an important role in neural transmission and ganglioside structure in synaptogenesis.[7] More than 50 kinds of sialic acid are known, all of which can be obtained from a molecule of neuraminic acid by substituting its amino group or one of its hydroxyl groups.[1] In general, the amino group bears either an acetyl or a glycolyl group, but other modifications have been described. These modifications along with linkages have shown to be tissue specific and developmentally regulated expressions, so some of them are only found on certain types of glycoconjugates in specific cells.[8] The hydroxyl substituents may vary considerably; acetyl, lactyl, methyl, sulfate, and phosphate groups have been found.[10]
- ^ a b Varki, Ajit; Roland Schauer (2008). "Sialic Acids". in Essentials of Glycobiology. Cold Spring Harbor Press. pp. Ch. 14. ISBN 9780879697709.
- ^ Wagstaff, Ben (2018). "Identification of a Kdn biosynthesis pathway in the haptophyte Prymnesium parvum suggests widespread sialic acid biosynthesis among microalgae". Journal of Biological Chemistry. 293 (42): 16277–16290. doi:10.1074/jbc.RA118.004921. PMC 6200933. PMID 30171074.
- ^ Ajit, Varki (2017). "Sialic Acids and Other Nonulosonic Acids". Sialic acids and other nonulosonic acids." Essentials of Glycobiology. Cold Spring Harbor Laboratory Press. doi:10.1101/glycobiology.3e.015 (inactive 1 November 2024). PMID 28876847.
{{cite book}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Kleikamp, Hugo (2020). "Tackling the chemical diversity of microbial nonulosonic acids – a universal large-scale survey approach". Chemical Science. 11 (11): 3074–3080. doi:10.1039/c9sc06406k. PMC 8157484. PMID 34122812.
- ^ Lewis, Amanda (2009). "Innovations in host and microbial sialic acid biosynthesis revealed by phylogenomic prediction of nonulosonic acid structure". Proceedings of the National Academy of Sciences. 106 (32): 13552–13557. Bibcode:2009PNAS..10613552L. doi:10.1073/pnas.0902431106. PMC 2726416. PMID 19666579.
- ^ Schauer, Roland (2018). "Exploration of the Sialic Acid World". Adv Carbohydr Chem Biochem. Advances in Carbohydrate Chemistry and Biochemistry. 75 (75): 1–213. doi:10.1016/bs.accb.2018.09.001. ISBN 9780128152027. PMC 7112061. PMID 30509400.
- ^ a b Wang, B.; Brand-Miller, J. (2003). "The role and potential of sialic acid in human nutrition". European Journal of Clinical Nutrition. 57 (11): 1351–1369. doi:10.1038/sj.ejcn.1601704. PMID 14576748.
- ^ a b Mandal, C. (1990). "Sialic acid binding lectins". Experientia. 46 (5): 433–441. doi:10.1007/BF01954221. PMID 2189746. S2CID 27075067.
- ^ Varki, Ajit; Roland Schauer (2008). "Sialic Acids". in Essentials of Glycobiology. Cold Spring Harbor Press. pp. Ch. 14. ISBN 9780879697709.
- ^ Schauer R. (2000). "Achievements and challenges of sialic acid research". Glycoconj. J. 17 (7–9): 485–499. doi:10.1023/A:1011062223612. PMC 7087979. PMID 11421344.