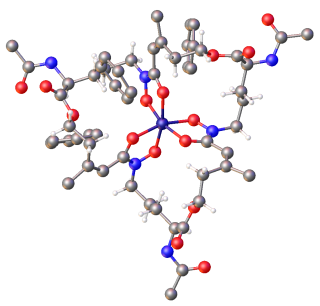
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron.[2][3][4][5] Although a widening range of siderophore functions is now being appreciated,[6] siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
- ^ Hossain MB, Eng-Wilmot DL, Loghry RA, an der Helm D (1980). "Circular Dichroism, Crystal Structure, and Absolute Configuration of the Siderophore Ferric N,N',N"-Triacetylfusarinine, FeC39H57N6O15". Journal of the American Chemical Society. 102 (18): 5766–5773. doi:10.1021/ja00538a012.
- ^ Neilands JB (November 1995). "Siderophores: structure and function of microbial iron transport compounds". The Journal of Biological Chemistry. 270 (45): 26723–6. doi:10.1074/jbc.270.45.26723. PMID 7592901.
- ^ Hider RC, Kong X (May 2010). "Chemistry and biology of siderophores". Natural Product Reports. 27 (5): 637–57. doi:10.1039/b906679a. PMID 20376388. S2CID 36973725.
- ^ Crosa JH, Mey AR, Payne SM, eds. (2004). Iron Transport in Bacteria. ASM Press. ISBN 978-1-55581-292-8.
- ^ Cornelis P, Andrews SC, eds. (2010). Iron Uptake and Homeostasis in Microorganisms. Caister Academic Press. ISBN 978-1-904455-65-3.
- ^ Johnstone TC, Nolan EM (April 2015). "Beyond iron: non-classical biological functions of bacterial siderophores". Dalton Transactions. 44 (14): 6320–39. doi:10.1039/C4DT03559C. PMC 4375017. PMID 25764171.