| |
| Names | |
|---|---|
| IUPAC name
Silver(I) azide
| |
| Other names
Argentous azide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.173 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| AgN3 | |
| Molar mass | 149.888 g/mol |
| Appearance | colorless crystals |
| Density | 4.42 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) explosive |
| Boiling point | decomposes |
| Solubility in other solvents | 2.0×10−8 g/L |
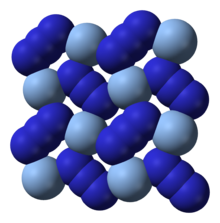
| Structure | |
| Orthorhombic oI16[1] | |
| Ibam, No 72 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Very toxic, explosive |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.
- ^ Marr H.E. III.; Stanford R.H. Jr. (1962). "The unit-cell dimensions of silver azide". Acta Crystallographica. 15 (12): 1313–1314. Bibcode:1962AcCry..15.1313M. doi:10.1107/S0365110X62003497.
