 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɪtəˈɡlɪptɪn/ |
| Trade names | Januvia, Zituvio, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606023 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 87% |
| Protein binding | 38% |
| Metabolism | Liver (CYP3A4- and CYP2C8-mediated) |
| Elimination half-life | 8 to 14 h[6] |
| Excretion | Kidney (80%)[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.948 |
| Chemical and physical data | |
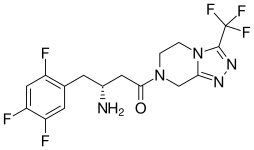
| Formula | C16H15F6N5O |
| Molar mass | 407.320 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Sitagliptin, sold under the brand name Januvia among others, is an anti-diabetic medication used to treat type 2 diabetes.[7] In the United Kingdom it is listed as less preferred than metformin or a sulfonylurea.[8] It is taken by mouth.[7] It is also available in the fixed-dose combination medication sitagliptin/metformin (Janumet, Janumet XR).[7]
Common side effects include headaches, swelling of the legs, and upper respiratory tract infections.[7] Serious side effects may include angioedema, low blood sugar, kidney problems, pancreatitis, and joint pain.[7] Whether use in pregnancy or breastfeeding is safe is unclear.[9] It is in the dipeptidyl peptidase-4 (DPP-4) inhibitor class and works by increasing the production of insulin and decreasing the production of glucagon by the pancreas.[7]
Sitagliptin was developed by Merck & Co. and approved for medical use in the United States in 2006.[7] In 2021, it was the 83rd most commonly prescribed medication in the United States, with more than 8 million prescriptions.[10][11] It is available as a generic medication in Canada but not the United States.[12][13]
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved March 24, 2024.
- ^ "Januvia- sitagliptin tablet, film coated". DailyMed. Archived from the original on October 27, 2021. Retrieved October 15, 2021.
- ^ "Zituvio- sitagliptin tablet". DailyMed. November 1, 2023. Retrieved December 25, 2023.
- ^ "Zituvio- sitagliptin tablet". DailyMed. November 1, 2023. Retrieved December 25, 2023.
- ^ "Januvia EPAR". European Medicines Agency. September 17, 2018. Archived from the original on October 23, 2021. Retrieved October 15, 2021.
- ^ a b Herman GA, Stevens C, van Dyck K, Bergman A, Yi B, De Smet M, et al. (December 2005). "Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses". Clinical Pharmacology and Therapeutics. 78 (6): 675–688. doi:10.1016/j.clpt.2005.09.002. PMID 16338283. S2CID 20935646.
- ^ a b c d e f g "Sitagliptin Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on March 4, 2016. Retrieved March 3, 2019.
- ^ Cite error: The named reference
BNF76was invoked but never defined (see the help page). - ^ "Sitagliptin Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on March 6, 2019. Retrieved March 3, 2019.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on January 15, 2024. Retrieved January 14, 2024.
- ^ "Sitagliptin - Drug Usage Statistics". ClinCalc. Retrieved January 14, 2024.
- ^ "Generic Januvia Availability". Drugs.com. Retrieved December 1, 2023.
- ^ "JAMP Pharma Group receives Health Canada approval for PrJAMP Sitagliptin, a new generic alternative for the treatment of type 2 diabetes" (Press release). JAMP Pharma. January 6, 2023. Retrieved June 19, 2023 – via Newswire.