| |
| Names | |
|---|---|
| IUPAC name
Sodium bifluoride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.014.190 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 2439 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
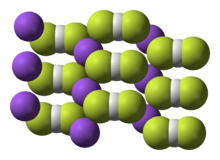
| Na[HF2] | |
| Molar mass | 61.995 g·mol−1 |
| Appearance | white solid |
| Density | 2.08 g/cm3 |
| Melting point | 160 °C (320 °F; 433 K) (decomposes) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H301, H314 | |
| P260, P264, P270, P280, P301+P310, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation (Na+) and bifluoride anion ([HF2]−). It is a white, water-soluble solid that decomposes upon heating .[2] Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell.[3] Sodium bifluoride has a number of applications in industry.