| |

| |
 Unit cell of sodium chlorate
| |
| Names | |
|---|---|
| IUPAC name
Sodium chlorate
| |
| Other names
Sodium chlorate(V)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.989 |
| EC Number |
|
| KEGG | |
| MeSH | Sodium+chlorate |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1495, 2428 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NaClO3 | |
| Molar mass | 106.44 g mol−1 |
| Appearance | Colorless or white solid, hygroscopic |
| Odor | Odorless |
| Density | 2.49 g/cm3 (15 °C)[1] 2.54 g/cm3 (20.2 °C)[2] |
| Melting point | 248–261 °C (478–502 °F; 521–534 K) |
| Boiling point | 300–400 °C (572–752 °F; 573–673 K) decomposes[1] |
| 79 g/100 mL (0 °C) 89 g/100 mL (10 °C) 105.7 g/100 mL (25 °C) 125 g/100 mL (40 °C) 220.4 g/100 mL (100 °C)[3] | |
| Solubility | Soluble in glycerol, hydrazine, methanol Slightly soluble in ethanol, ammonia[1] |
| Solubility in acetone | Sparingly soluble[1] |
| Solubility in glycerol | 20 g/100 g (15.5 °C)[1] |
| Solubility in ethanol | 14.7 g/100 g[1] |
| Vapor pressure | <0.35 mPa[2] |
| −34.7·10−6 cm3/mol | |
Refractive index (nD)
|
1.515 (20 °C)[4] |
| Structure[5] | |
| cubic | |
| P213 | |
a = 6.57584 Å
| |
Formula units (Z)
|
4 |
| Thermochemistry | |
Heat capacity (C)
|
104.6 J/mol·K[1] |
Std molar
entropy (S⦵298) |
129.7 J/mol·K[1] |
Std enthalpy of
formation (ΔfH⦵298) |
-365.4 kJ/mol[1] |
Gibbs free energy (ΔfG⦵)
|
-275 kJ/mol[1] |
| Hazards | |
| GHS labelling: | |
   [6] [6]
| |
| Danger | |
| H271, H302, H411[6] | |
| P220, P273[6] | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
600 mg/kg (rats, oral) 700 mg/kg (dogs, oral)[1] |
| Safety data sheet (SDS) | ICSC 1117 |
| Related compounds | |
Other anions
|
Sodium chloride Sodium hypochlorite Sodium chlorite Sodium perchlorate Sodium bromate Sodium iodate |
Other cations
|
Ammonium chlorate Potassium chlorate Barium chlorate |
Related compounds
|
Chloric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
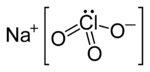
Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen[4] and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper.[7]
- ^ a b c d e f g h i j k "Sodium chlorate".
- ^ a b "GPS Safety Summary of Sodium Chlorate" (PDF). arkema.com. Arkema. Archived from the original (PDF) on 2014-05-25. Retrieved 2014-05-25.
- ^ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand.
- ^ a b CID 516902 from PubChem
- ^ S. C. Abrahams, J. L. Bernstein (1977). "Remeasurement of Optically Active NaClO3 and NaBrO3". Acta Crystallographica. B33 (11): 3601–3604. Bibcode:1977AcCrB..33.3601A. doi:10.1107/S0567740877011637.
- ^ a b c Sigma-Aldrich Co., Sodium chlorate. Retrieved on 2022-02-21.
- ^ Cite error: The named reference
Ullmannwas invoked but never defined (see the help page).
