| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium dodecyl sulfate | |
| Other names
Sodium monododecyl sulfate; Sodium lauryl sulfate; Sodium monolauryl sulfate; Sodium dodecanesulfate; dodecyl alcohol, hydrogen sulfate, sodium salt; n-dodecyl sulfate sodium; Sulfuric acid monododecyl ester sodium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.263 |
| E number | E487 (thickeners, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H25NaSO4 | |
| Molar mass | 288.372 g/mol |
| Appearance | white or cream-colored solid |
| Odor | odorless |
| Density | 1.01 g/cm3 |
| Melting point | 206 °C (403 °F; 479 K) |
| Surface tension: | |
| 8.2 mM at 25 °C[1] | |
Refractive index (nD)
|
1.461 |
| Pharmacology | |
| A06AG11 (WHO) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1288 mg/kg (rat, oral) |
| Related compounds | |
Other anions
|
Sodium laureth sulfate Sodium myreth sulfate |
Other cations
|
Ammonium lauryl sulfate Potassium lauryl sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
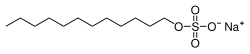
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula CH3(CH2)11OSO3Na and structure H3C−(CH2)11−O−S(=O)2−O−Na+. It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations.[2]
- ^ P. Mukerjee, P. & Mysels, K. J. (1971), "Critical Micelle Concentration of Aqueous Surfactant Systems," NSRDS-NBS 36, Washington, DC: US. Government Printing Office.[full citation needed][page needed]
- ^ Cite error: The named reference
UllmannSurfwas invoked but never defined (see the help page).