| |
| Names | |
|---|---|
| IUPAC name
Pentasodium triphosphate
| |
| Other names
sodium tripolyphosphate, polygon, STPP
| |
| Identifiers | |
| ECHA InfoCard | 100.028.944 |
| E number | E451 (thickeners, ...) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
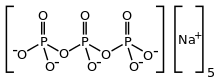
| Na5P3O10 | |
| Molar mass | 367.864 g/mol |
| Appearance | white powder |
| Density | 2.52 g/cm3 |
| Melting point | 622 °C (1,152 °F; 895 K) |
| 14.5 g/100 mL (25 °C) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | ICSC 1469 |
| Related compounds | |
Other anions
|
Trisodium phosphate Tetrasodium pyrophosphate Sodium hexametaphosphate |
Other cations
|
Pentapotassium triphosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium triphosphate (STP), also sodium tripolyphosphate (STPP), or tripolyphosphate (TPP),[1]) is an inorganic compound with formula Na5P3O10. It is the sodium salt of the polyphosphate penta-anion, which is the conjugate base of triphosphoric acid. It is produced on a large scale as a component of many domestic and industrial products, especially detergents. Environmental problems associated with eutrophication are attributed to its widespread use.[2]
- ^ Complexing agents, Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products, Danish Environmental Protection Agency Archived 2017-08-24 at the Wayback Machine, Accessed 2008-07-15
- ^ Cite error: The named reference
GEwas invoked but never defined (see the help page).
