| |

| |
| Names | |
|---|---|
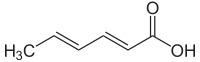
| Preferred IUPAC name
(2E,4E)-Hexa-2,4-dienoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.427 |
| E number | E200 (preservatives) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H8O2 | |
| Molar mass | 112.128 g·mol−1 |
| Density | 1.204 g/cm3 |
| Melting point | 135 °C (275 °F; 408 K) |
| Boiling point | 228 °C (442 °F; 501 K) |
| 1.6 g/L at 20 °C | |
| Acidity (pKa) | 4.76 at 25 °C |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sorbic acid, or 2,4-hexadienoic acid, is a natural organic compound used as a food preservative. It has the chemical formula CH3(CH)4CO2H and the structure H3C−CH=CH−CH=CH−C(=O)OH. It is a colourless solid that is slightly soluble in water and sublimes readily. It was first isolated from the unripe berries of the Sorbus aucuparia (rowan tree), hence its name.[1]
