| Square pyramidal molecular geometry | |
|---|---|
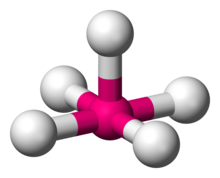 | |
| Examples | Chlorine pentafluoride (ClF5), MnCl2−5 |
| Point group | C4v |
| Coordination number | 5 |
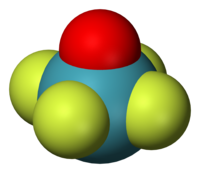
Square pyramidal geometry describes the shape of certain chemical compounds with the formula ML5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably [Ni(CN)5]3−.[1]
- ^ Spiro, Thomas G.; Terzis, Aristides; Raymond, Kenneth N. (1970). "Structure of Ni(CN)3−
5. Raman, infrared, and x-ray crystallographic evidence". Inorg. Chem. 9 (11): 2415. doi:10.1021/ic50093a006.