Synthesis-dependent strand annealing (SDSA) is a major mechanism of homology-directed repair of DNA double-strand breaks (DSBs). Although many of the features of SDSA were first suggested in 1976,[1] the double-Holliday junction model proposed in 1983[2] was favored by many researchers. In 1994, studies of double-strand gap repair in Drosophila were found to be incompatible with the double-Holliday junction model, leading researchers to propose a model they called synthesis-dependent strand annealing.[3] Subsequent studies of meiotic recombination in S. cerevisiae found that non-crossover products appear earlier than double-Holliday junctions or crossover products, challenging the previous notion that both crossover and non-crossover products are produced by double-Holliday junctions and leading the authors to propose that non-crossover products are generated through SDSA.[4]
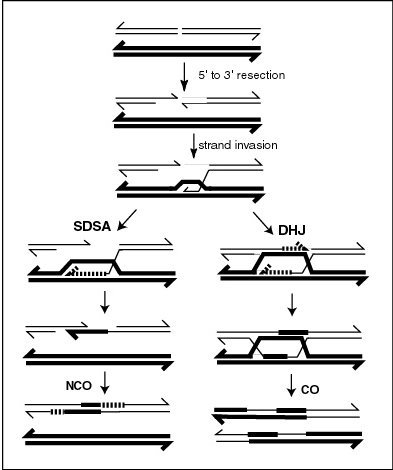
In the accompanying Figure, the first step labeled “5’ to 3’ resection” shows the formation of a 3’ ended single DNA strand that in the next step invades a homologous DNA duplex. RNA polymerase III is reported to catalyze formation of a transient RNA-DNA hybrid at double-strand breaks as an essential intermediate step in the repair of the breaks by homologous recombination.[5] Formation of the RNA-DNA hybrid would protect the invading single-stranded DNA from degradation. After the transient RNA-DNA hybrid intermediate is formed the RNA strand is replaced by the Rad51 protein which catalyzes the subsequent stage of strand invasion.
In the SDSA model, repair of double-stranded breaks occurs without the formation of a double Holliday junction, so that the two processes of homologous recombination are identical until just after D-loop formation.[6] In yeast, the D-loop is formed by strand invasion with the help of proteins Rad51 and Rad52,[7] and is then acted on by DNA helicase Srs2 to prevent formation of the double Holliday junction in order for the SDSA pathway to occur.[8] The invading 3' strand is thus extended along the recipient homologous DNA duplex by DNA polymerase in the 5' to 3' direction, so that the D-loop physically translocates – a process referred to as bubble migration DNA synthesis.[9] The resulting single Holliday junction then slides down the DNA duplex in the same direction in a process called branch migration, displacing the extended strand from the template strand. This displaced strand pops up to form a 3' overhang in the original double-stranded break duplex, which can then anneal to the opposite end of the original break through complementary base pairing. Thus DNA synthesis fills in gaps left over from annealing, and extends both ends of the still present single stranded DNA break, ligating all remaining gaps to produce recombinant non-crossover DNA.[10]
SDSA is unique in that D-loop translocation results in conservative rather than semiconservative replication, as the first extended strand is displaced from its template strand, leaving the homologous duplex intact. Therefore, although SDSA produces non-crossover products because flanking markers of heteroduplex DNA are not exchanged, gene conversion may occur, wherein nonreciprocal genetic transfer takes place between two homologous sequences.[11]
- ^ Resnick MA (June 1976). "The repair of double-strand breaks in DNA; a model involving recombination". Journal of Theoretical Biology. 59 (1): 97–106. Bibcode:1976JThBi..59...97R. doi:10.1016/s0022-5193(76)80025-2. PMID 940351.
- ^ Szostak JW, Orr-Weaver TL, Rothstein R, Stahl FW (May 1983). "The double-strand-break repair model for recombination". Cell. 33 (1): 25–35. doi:10.1016/0092-8674(83)90331-8. PMID 6380756. S2CID 39590123.
- ^ Nassif N, Penney J, Pal S, Engels WR, Gloor GB (March 1994). "Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair". Molecular and Cellular Biology. 14 (3): 1613–25. doi:10.1128/mcb.14.3.1613. PMC 358520. PMID 8114699.
- ^ Allers T, Lichten M (July 2001). "Differential timing and control of noncrossover and crossover recombination during meiosis". Cell. 106 (1): 47–57. doi:10.1016/s0092-8674(01)00416-0. PMID 11461701.
- ^ Liu S, Hua Y, Wang J, Li L, Yuan J, Zhang B, Wang Z, Ji J, Kong D. RNA polymerase III is required for the repair of DNA double-strand breaks by homologous recombination. Cell. 2021 Mar 4;184(5):1314-1329.e10. doi: 10.1016/j.cell.2021.01.048. Epub 2021 Feb 23. PMID: 33626331
- ^ McMahill MS, Sham CW, Bishop DK (November 2007). "Synthesis-dependent strand annealing in meiosis". PLOS Biology. 5 (11): e299. doi:10.1371/journal.pbio.0050299. PMC 2062477. PMID 17988174.
- ^ Dupaigne P, Le Breton C, Fabre F, Gangloff S, Le Cam E, Veaute X (February 2008). "The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination". Molecular Cell. 29 (2): 243–54. doi:10.1016/j.molcel.2007.11.033. PMID 18243118.
- ^ Miura T, Yamana Y, Usui T, Ogawa HI, Yamamoto MT, Kusano K (May 2012). "Homologous recombination via synthesis-dependent strand annealing in yeast requires the Irc20 and Srs2 DNA helicases". Genetics. 191 (1): 65–78. doi:10.1534/genetics.112.139105. PMC 3338270. PMID 22367032.
- ^ Formosa T, Alberts BM (December 1986). "DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins". Cell. 47 (5): 793–806. doi:10.1016/0092-8674(86)90522-2. PMID 3022939. S2CID 37903641.
- ^ Helleday T, Lo J, van Gent DC, Engelward BP (July 2007). "DNA double-strand break repair: from mechanistic understanding to cancer treatment". DNA Repair. 6 (7): 923–35. doi:10.1016/j.dnarep.2007.02.006. PMID 17363343.
- ^ Maher RL, Branagan AM, Morrical SW (October 2011). "Coordination of DNA replication and recombination activities in the maintenance of genome stability". Journal of Cellular Biochemistry. 112 (10): 2672–82. doi:10.1002/jcb.23211. PMC 3178728. PMID 21647941.