 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prograf, Advagraf, Protopic, others |
| Other names | FK-506, fujimycin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601117 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical, by mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 24% (5–67%), less after eating food rich in fat |
| Protein binding | ≥98.8% |
| Metabolism | Liver CYP3A4, CYP3A5 |
| Elimination half-life | 11.3 h for transplant patients (range 3.5–40.6 h) |
| Excretion | Mostly fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.155.367 |
| Chemical and physical data | |
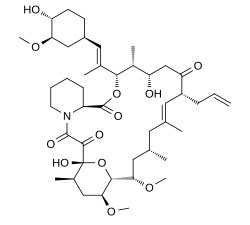
| Formula | C44H69NO12 |
| Molar mass | 804.031 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tacrolimus, sold under the brand name Prograf among others, is an immunosuppressive drug. After allogenic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, tacrolimus is given. The drug can also be sold as a topical medication in the treatment of T cell-mediated diseases such as eczema and psoriasis. For example, it is prescribed for severe refractory uveitis after a bone marrow transplant, exacerbations of minimal change disease, Kimura's disease, and vitiligo. It can be used to treat dry eye syndrome in cats and dogs.[7][8]
Tacrolimus inhibits calcineurin, which is involved in the production of interleukin-2, a molecule that promotes the development and proliferation of T cells, as part of the body's learned (or adaptive) immune response.
Chemically, it is a macrolide lactone[9] that was first discovered in 1987, from the fermentation broth of a Japanese soil sample that contained the bacterium Streptomyces tsukubensis. It is on the World Health Organization's List of Essential Medicines.[10] In 2021, it was the 296th most commonly prescribed medication in the United States, with more than 500,000 prescriptions.[11][12]
- ^ "Tacrolimus Use During Pregnancy". Drugs.com. 3 October 2019. Retrieved 29 April 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Drug and medical device highlights 2019: Helping you maintain and improve your health". Health Canada. 18 November 2020. Retrieved 28 March 2024.
- ^ "Prograf- tacrolimus capsule, gelatin coated Prograf- tacrolimus injection, solution Prograf- tacrolimus granule, for suspension". DailyMed. Retrieved 16 July 2021.
- ^ "Advagraf EPAR". European Medicines Agency. 17 September 2018. Retrieved 16 July 2021.
- ^ "Protopic EPAR". European Medicines Agency. 17 September 2018. Retrieved 16 July 2021.
- ^ Berdoulay A, English RV, Nadelstein B (2005). "Effect of topical 0.02% tacrolimus aqueous suspension on tear production in dogs with keratoconjunctivitis sicca". Veterinary Ophthalmology. 8 (4): 225–232. doi:10.1111/j.1463-5224.2005.00390.x. PMID 16008701.
- ^ "Tacrolimus for Dogs and Cats".
- ^ Baldo A, Cafiero M, Di Caterino P, Di Costanzo L (January 2009). "Tacrolimus ointment in the management of atopic dermatitis". Clinical, Cosmetic and Investigational Dermatology. 2: 1–7. doi:10.2147/ccid.s3378. PMC 3047924. PMID 21436963.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Tacrolimus - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.