 | |
| Clinical data | |
|---|---|
| Trade names | Saflutan, Taflotan, Zioptan |
| AHFS/Drugs.com | Multum Consumer Information |
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation |
| Onset of action | 2–4 hrs |
| Duration of action | ≥ 24 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.745 |
| Chemical and physical data | |
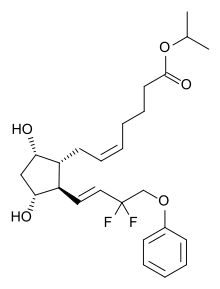
| Formula | C25H34F2O5 |
| Molar mass | 452.539 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tafluprost (trade names Taflotan by Santen Pharmaceutical, Zioptan by Merck in the US and Saflutan by Mundipharma in Australia) is a prostaglandin analogue. It is used topically (as eye drops) to control the progression of open-angle glaucoma and in the management of ocular hypertension, alone or in combination with other medication. It reduces intraocular pressure by increasing the outflow of aqueous fluid from the eyes.[2][3]