| |
| Names | |
|---|---|
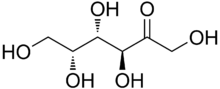
| IUPAC name
D-lyxo-Hex-2-ulose[1]
| |
| Systematic IUPAC name
(3S,4S,5R)-1,3,4,5,6-Pentahydroxy-hexan-2-one | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.001.612 |
| E number | E963 (glazing agents, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
| Appearance | White solid |
| Melting point | 133 to 135 °C (271 to 275 °F; 406 to 408 K) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tagatose is a hexose monosaccharide. It is found in small quantities in a variety of foods, and has attracted attention as an alternative sweetener.[2] It is often found in dairy products, because it is formed when milk is heated. It is similar in texture and appearance to sucrose (table sugar)[3]:215 and is 92% as sweet,[3]:198 but with only 38% of the calories.[3]:209 Tagatose is generally recognized as safe by the Food and Agriculture Organization and the World Health Organization, and has been since 2001. Since it is metabolized differently from sucrose, tagatose has a minimal effect on blood glucose and insulin levels. Tagatose is also approved as a tooth-friendly ingredient for dental products. Consumption of more than about 30 grams of tagatose in a dose may cause gastric disturbance in some people, as it is mostly processed in the large intestine, similar to soluble fiber.[3]:214
- ^ iupac.qmul.ac.uk/2carb/10.html
- ^ Mu, Wanmeng; Hassanin, Hinawi A. M.; Zhou, Leon; Jiang, Bo (2018). "Chemistry Behind Rare Sugars and Bioprocessing". Journal of Agricultural and Food Chemistry. 66 (51): 13343–13345. doi:10.1021/acs.jafc.8b06293. PMID 30543101. S2CID 56145019.
- ^ a b c d Alternative sweeteners. Nabors, Lyn O'Brien, 1943- (4th ed.). Boca Raton, FL: CRC Press. 2012. ISBN 978-1-4398-4615-5. OCLC 760056415.
{{cite book}}: CS1 maint: others (link)
