| |

| |
| Names | |
|---|---|
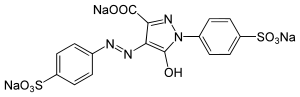
| IUPAC name
Trisodium 5-hydroxy-1-(4-sulfonatophenyl)-4-[(E)-(4-sulfonatophenyl)diazenyl]-1H-pyrazole-3-carboxylate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.016.091 |
| E number | E102 (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H9N4Na3O9S2 | |
| Molar mass | 534.36 g·mol−1 |
| 20 g/100 mL | |
| Solubility | 18 g/100 mL in glycerol, negligible in ethanol |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tartrazine is a synthetic lemon yellow azo dye primarily used as a food coloring.[1][2][3][4] It is also known as E number E102, C.I. 19140, FD&C Yellow 5, Yellow 5 Lake, Acid Yellow 23, Food Yellow 4, and trisodium 1-(4-sulfonatophenyl)-4-(4-sulfonatophenylazo)-5-pyrazolone-3-carboxylate.[5]
Tartrazine is a commonly used coloring agent all over the world, mainly for yellow, and can also be used with brilliant blue FCF (FD&C Blue 1, E133) or green S (E142) to produce various green shades. It serves as a dye for wool and silks, a colorant in food, drugs and cosmetics and an adsorption-elution indicator for chloride estimations in biochemistry.
- ^ Food Standards Australia New Zealand. "Food Additives- Numerical List". Archived from the original on June 25, 2009. Retrieved 2 December 2009.
- ^ Current EU approved additives and their E Numbers, Food Standards Agency website, retrieved 15 Dec 2011
- ^ "Food Dyes". Center for Science in the Public Interest. Archived from the original on 6 July 2016. Retrieved 8 March 2013.
- ^ "What is Food Coloring Made Of?". WiseGeek. Retrieved 8 March 2013.
- ^ "Acid Yellow 23". ChemBlink, an online database of chemicals from around the world.
