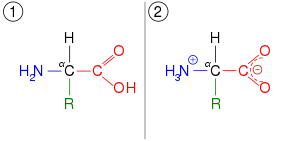
In chemistry, tautomers (/ˈtɔːtəmər/)[1] are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.[2][3][4][5] The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. The term is derived from Ancient Greek ταὐτό (tautó) 'the same' and μέρος (méros) 'part'.
Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties,[6] whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the "average" of the idealized, hypothetical geometries implied by these resonance forms.
- ^ "tautomer". Oxford Dictionaries - English. Archived from the original on 2018-02-19.
- ^ Antonov L (2013). Tautomerism: Methods and Theories (1st ed.). Weinheim, Germany: Wiley-VCH. ISBN 978-3-527-33294-6.
- ^ Antonov L (2016). Tautomerism: Concepts and Applications in Science and Technology (1st ed.). Weinheim, Germany: Wiley-VCH. ISBN 978-3-527-33995-2.
- ^ Smith, Michael B. (19 February 2020). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. Wiley. pp. 96–103. ISBN 9781119371809.
- ^ Katritzky AR, Elguero J, et al. (1976). The Tautomerism of heterocycles. New York: Academic Press. ISBN 978-0-12-020651-3.
- ^ Smith, Kyle T.; Young, Sherri C.; DeBlasio, James W.; Hamann, Christian S. (27 January 2016). "Measuring Structural and Electronic Effects on Keto–Enol Equilibrium in 1,3-Dicarbonyl Compounds". Journal of Chemical Education. 93 (4): 790–794. Bibcode:2016JChEd..93..790S. doi:10.1021/acs.jchemed.5b00170.