 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Kerydin |
| Other names | AN2690 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614049 |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.130 |
| Chemical and physical data | |
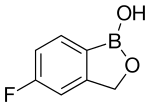
| Formula | C7H6BFO2 |
| Molar mass | 151.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tavaborole, sold under the brand name Kerydin, is a topical antifungal medication for the treatment of onychomycosis, a fungal infection of the nail and nail bed with a complete clearance rate of 6-7% and partial clearance rate of 23-24% in individuals whose “infection border does not reach the cuticle at the base of the large toenail.”[1] Tavaborole was approved by the US FDA in July 2014.[2] The medication inhibits an essential fungal enzyme, leucyl-tRNA synthetase, that is required for protein synthesis. The inhibition of protein synthesis leads to termination of cell growth and then cell death, eliminating the fungal infection.
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ "FDA Approves Anacor Pharmaceuticals' KERYDIN™ (Tavaborole) Topical Solution, 5% for the Treatment of Onychomycosis of the Toenails". Market Watch. July 8, 2014.