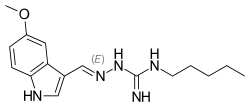 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zelnorm, Zelmac |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10% |
| Protein binding | 98% |
| Metabolism | Gastric and hepatic |
| Elimination half-life | 11 ± 5 hours |
| Excretion | Fecal and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.158.793 |
| Chemical and physical data | |
| Formula | C16H23N5O |
| Molar mass | 301.394 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tegaserod is a 5-HT4 agonist manufactured by Novartis and sold under the names Zelnorm and Zelmac for the management of irritable bowel syndrome and constipation.[1] Approved by the FDA in 2002, it was subsequently removed from the market in 2007 due to FDA concerns about possible adverse cardiovascular effects. Before then, it was the only drug approved by the United States Food and Drug Administration to help relieve the abdominal discomfort, bloating, and constipation associated with irritable bowel syndrome. Its use was also approved to treat chronic idiopathic constipation.[2]
- ^ "New Data for Zelnorm". Archived from the original on December 9, 2007. Retrieved March 30, 2007.
- ^ "FDA approves first treatment for women with irritable-bowel syndrome". Food and Drug Administration. Archived from the original on February 5, 2007. Retrieved March 30, 2007.