 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌtaɪkoʊˈpleɪnɪn/ TY-koh-PLAY-nin |
| Trade names | Targocid |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% (given IM) |
| Protein binding | 90% to 95% |
| Metabolism | Nil |
| Elimination half-life | 70 to 100 hours |
| Excretion | Kidney (97% unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | Variable |
| Molar mass | 1564.3 to 1907.7 g/mol |
| Melting point | 260 °C (500 °F) (dec.) |
| |
| | |
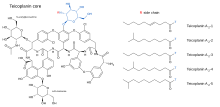
Teicoplanin is an semisynthetic glycopeptide antibiotic with a spectrum of activity similar to vancomycin. Its mechanism of action is to inhibit bacterial cell wall[3] peptidoglycan[4] synthesis. It is used in the prophylaxis and treatment of serious infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and Enterococcus faecalis.[3]
Teicoplanin is marketed by Sanofi-Aventis under the trade name Targocid. Other trade names include Ticocin marketed by Cipla(India).
Oral teicoplanin has been demonstrated to be effective in the treatment of pseudomembranous colitis and Clostridioides difficile-associated diarrhoea, with comparable efficacy with vancomycin.[5]
Its strength is considered to be due to the length of the hydrocarbon chain.[6]
Teicoplanin is produced by the so-called "rare" actinobacterium Actinoplanes teichomyceticus ATCC 31121,[7] belonging to the Micromonosporaceae family. Biosynthetic pathway leading to teicoplanin, as well as the regulatory circuit governing the biosynthesis, were studied intensively in recent years allowing to construct an integrated model of the biosynthesis.[8]
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 30 March 2024.
- ^ Human Medicines Evaluation Division (10 December 2020). "Active substance: teicoplanin" (PDF). List of nationally authorised medicinal products. European Medicines Agency.
- ^ a b Reynolds PE (November 1989). "Structure, biochemistry and mechanism of action of glycopeptide antibiotics". European Journal of Clinical Microbiology & Infectious Diseases. 8 (11): 943–950. doi:10.1007/BF01967563. PMID 2532132. S2CID 21551939.
- ^ "Teicoplanin Susceptibility and Minimum Inhibitory Concentration (MIC) Data" (PDF). TOKU-E.
- ^ de Lalla F, Nicolin R, Rinaldi E, Scarpellini P, Rigoli R, Manfrin V, et al. (October 1992). "Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea". Antimicrobial Agents and Chemotherapy. 36 (10): 2192–2196. doi:10.1128/AAC.36.10.2192. PMC 245474. PMID 1444298.
- ^ Gilpin M, Milner P (1997). "Resisting changes -- Over the past 40 years the glycopeptide antibiotics have played a crucial role in treating bacterial infections. But how long can it continue?". Royal Society of Chemistry. Archived from the original on 2002-12-21. Retrieved 2006-10-15. - includes picture of Teicoplanin's structure.
- ^ "Actinoplanes teichomyceticus Parenti et al". ATCC (American Type Culture Collection).
- ^ Yushchuk O, Ostash B, Truman AW, Marinelli F, Fedorenko V (April 2020). "Teicoplanin biosynthesis: unraveling the interplay of structural, regulatory, and resistance genes". Applied Microbiology and Biotechnology. 104 (8): 3279–3291. doi:10.1007/s00253-020-10436-y. PMID 32076781. S2CID 211170874.