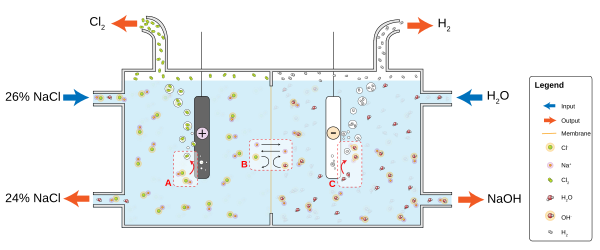
A diagram showing the chloralkali process, a major industry expected to be worth over $80 billion globally by 2017. At the anode (A), chloride (Cl-) is oxidized to chlorine. The ion-selective membrane (B) allows the counterion sodium (Na+) to freely flow across, but prevents anions such as hydroxide (OH-) and chloride from diffusing across. At the cathode (C), water is reduced to hydroxide and hydrogen gas. The net process is the electrolysis of an aqueous solution of NaCl into industrially useful products sodium hydroxide (NaOH) and chlorine gas.Illustration: John C