 | |
| Clinical data | |
|---|---|
| Trade names | Seldane, Triludan, Teldane |
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a600034 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 70% |
| Metabolism | Hepatic (CYP3A4) |
| Metabolites | Fexofenadine |
| Elimination half-life | 3.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.537 |
| Chemical and physical data | |
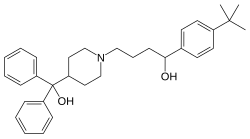
| Formula | C32H41NO2 |
| Molar mass | 471.685 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Terfenadine is an antihistamine formerly used for the treatment of allergic conditions. It was brought to market by Hoechst Marion Roussel (now Sanofi) and was marketed under various brand names, including Seldane in the United States, Triludan in the United Kingdom, and Teldane in Australia.[1] It was superseded by fexofenadine in the 1990s due to the risk of a particular type of disruption of the electrical rhythms of the heart (specifically cardiac arrhythmia caused by QT interval prolongation) and has been withdrawn from markets worldwide.[2]: 53
- ^ Cite error: The named reference
jama96was invoked but never defined (see the help page). - ^ Horak F (2010). "Antialergic and Vasoactive Drugs for Allergic Rhinitis Chapter 4". In Pawankar R, Holgate ST, Rosenwasser LJ (eds.). Allergy Frontiers:Therapy and Prevention. Vol. 5. Springer Science & Business Media. ISBN 978-4-431-99362-9.