 | |
| Clinical data | |
|---|---|
| Trade names | Forteo, Forsteo |
| Biosimilars | Bonsity,[1] Kauliv,[2] Livogiva,[3] Osnuvo,[4] Qutavina,[5] Sondelbay,[6] Teribone,[7] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603018 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Metabolism | Liver (nonspecific proteolysis) |
| Elimination half-life | Subcutaneous: 1 hour |
| Excretion | Kidney (metabolites) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.168.733 |
| Chemical and physical data | |
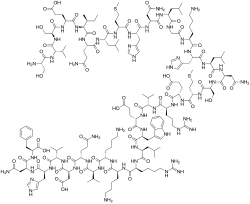
| Formula | C181H291N55O51S2 |
| Molar mass | 4117.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Teriparatide, sold under the brand name Forteo, is a form of parathyroid hormone (PTH) consisting of the first (N-terminus) 34 amino acids, which is the bioactive portion of the hormone.[13] It is an effective anabolic (promoting bone formation) agent[15] used in the treatment of some forms of osteoporosis.[13][16] Teriparatide is a recombinant human parathyroid hormone analog (PTH 1-34).[13] It has an identical sequence to the 34 N-terminal amino acids of the 84-amino acid human parathyroid hormone.[13]
- ^ Cite error: The named reference
FDA Bonsity approvalwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Kauliv EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Livogiva EPARwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Osnuvo SBDwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Qutavina EPARwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Sondelbay EPARwas invoked but never defined (see the help page). - ^ Lisbeth Tristan de Brea (18 September 2018). "Nota de Seguridad de Medicamentos" (PDF). Panama: Directora Nacional de Farmacia y Drogas. Archived (PDF) from the original on 4 December 2020. Retrieved 30 September 2018.
- ^ "Terrosa". Therapeutic Goods Administration (TGA). 26 May 2022. Archived from the original on 30 September 2022. Retrieved 7 July 2024.
- ^ "Teriparatide Use During Pregnancy". Drugs.com. 25 November 2019. Archived from the original on 27 October 2020. Retrieved 14 September 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Forteo teriparatide (rbe) 250 microgram solution for injection cartridge, Eli Lilly Australia Pty Ltd, CON-1240". Therapeutic Goods Administration (TGA). 17 June 2024. Archived from the original on 17 June 2024. Retrieved 17 June 2024.
- ^ "Ritosa teriparatide 250 microgram/mL solution for injection pre-filled cartridge (408423)". Therapeutic Goods Administration (TGA). 3 May 2024. Archived from the original on 17 June 2024. Retrieved 17 June 2024.
- ^ a b c d e "Forteo- teriparatide injection, solution". DailyMed. 29 April 2021. Archived from the original on 19 January 2022. Retrieved 8 March 2023.
- ^ Cite error: The named reference
Forsteo EPARwas invoked but never defined (see the help page). - ^ Riek AE, Towler DA (2011). "The pharmacological management of osteoporosis". Missouri Medicine. 108 (2): 118–23. PMC 3597219. PMID 21568234.
- ^ Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, et al. (November 2007). "Teriparatide or alendronate in glucocorticoid-induced osteoporosis". The New England Journal of Medicine. 357 (20): 2028–39. doi:10.1056/NEJMoa071408. PMID 18003959.