 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /tɛˈstɒstəroʊn/ teh-STOS-tə-rohn[1] |
| Trade names | AndroGel, Testim, TestoGel, others |
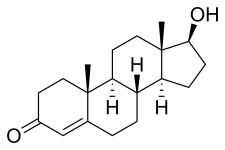
| Other names | Androst-4-en-17β-ol-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619028 |
| License data |
|
| Pregnancy category |
|
| Dependence liability | Moderate [3] |
| Addiction liability | Moderate [3] |
| Routes of administration | intramuscular, transdermal (gel, cream, patch, solution) oral, buccal, sublingual, intranasal, vaginal (cream, gel, suppository), rectal (suppository), subcutaneous injection (oil solution, aqueous suspension), subcutaneous implant (pellet) |
| Drug class | Androgen, anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: very low (due to extensive first pass metabolism) |
| Protein binding | 97.0–99.5% (to SHBG and albumin)[8] |
| Metabolism | Liver (mainly reduction and conjugation) |
| Elimination half-life | 2–4 hours[citation needed] |
| Excretion | Urine (90%), feces (6%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H28O2 |
| Molar mass | 288.431 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | +110.2° |
| Melting point | 155 °C (311 °F) |
| |
| |
| (verify) | |
Testosterone (T) is a medication and naturally occurring steroid hormone.[9] It is used to treat male hypogonadism, gender dysphoria, and certain types of breast cancer.[9][10] It may also be used to increase athletic ability in the form of doping.[9] It is unclear if the use of testosterone for low levels due to aging is beneficial or harmful.[11] Testosterone can be used as a gel or transdermal patch that is applied to the skin topically, intramuscular injection (IM), buccally (a tablet dissolved between the gum and cheek inside the mouth), or as an oral tablet (tablet swallowed by mouth).[9]
Common side effects of testosterone include acne, swelling, and breast enlargement in men.[9] Serious side effects may include liver toxicity, heart disease, and behavioral changes.[9] Women and children who are exposed may develop masculinization.[9] It is recommended that individuals with prostate cancer should not use the medication.[9] It can cause harm to the baby if used during pregnancy or breastfeeding.[9] Testosterone is in the androgen family of medications.[9]
Testosterone was first isolated in 1935, and approved for medical use in 1939.[12][13] Rates of use have increased three times in the United States between 2001 and 2011.[14] It is on the World Health Organization's List of Essential Medicines.[15] It is available as a generic medication.[9] In 2022, it was the 118th most commonly prescribed medication in the United States, with more than 5 million prescriptions.[16][17]
- ^ Testosterone. Oxford Dictionaries.
- ^ "Testosterone Use During Pregnancy". Drugs.com. August 20, 2019. Archived from the original on February 1, 2014. Retrieved January 8, 2020.
- ^ a b "Anabolic steroid misuse". nhs.uk. November 4, 2022. Retrieved July 12, 2024.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ Anvisa (March 31, 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published April 4, 2023). Archived from the original on August 3, 2023. Retrieved August 15, 2023.
- ^ Human Medicines Evaluation Division (September 1, 2022). "Active substance: testosterone (all formulations apart from topical use)" (PDF). List of nationally authorised medicinal products. European Medicines Agency. Archived (PDF) from the original on September 6, 2022. Retrieved September 6, 2022.
- ^ Human Medicines Evaluation Division (September 1, 2022). "Active substance: testosterone (topical use)" (PDF). List of nationally authorised medicinal products. European Medicines Agency. Archived (PDF) from the original on September 6, 2022. Retrieved September 6, 2022.
- ^ Melmed S, Polonsky KS, Larsen PR (November 11, 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 709, 711, 765. ISBN 978-0-323-34157-8. Archived from the original on April 14, 2019. Retrieved November 18, 2016.
- ^ a b c d e f g h i j k "Testosterone". Drugs.com. American Society of Health-System Pharmacists. December 4, 2015. Archived from the original on August 20, 2016. Retrieved September 3, 2016.
- ^ "List of Gender Dysphoria Medications (6 Compared)". Drugs.com. Archived from the original on April 26, 2020. Retrieved May 6, 2020.
- ^ Staff (March 3, 2015). "Testosterone Products: Drug Safety Communication – FDA Cautions About Using Testosterone Products for Low Testosterone Due to Aging; Requires Labeling Change to Inform of Possible Increased Risk of Heart Attack And Stroke". FDA. Archived from the original on March 5, 2015. Retrieved March 5, 2015.
- ^ Taylor WN (2002). Anabolic Steroids and the Athlete (2nd ed.). McFarland. p. 180. ISBN 978-0-7864-1128-3. Archived from the original on September 14, 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 481. ISBN 9783527607495. Archived from the original on August 23, 2022. Retrieved August 18, 2020.
- ^ Desroches B, Kohn TP, Welliver C, Pastuszak AW (April 2016). "Testosterone therapy in the new era of Food and Drug Administration oversight". Translational Andrology and Urology. 5 (2): 207–12. doi:10.21037/tau.2016.03.13. PMC 4837303. PMID 27141448.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on August 30, 2024. Retrieved August 30, 2024.
- ^ "Testosterone Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved August 30, 2024.