| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanetetracarbonitrile | |
| Other names
carbon tetracyanide; 2,2-dicyanomalononitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
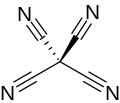
| C(CN)4 | |
| Molar mass | 116.083 g·mol−1 |
| Appearance | white crystals |
| Structure | |
| trigonal | |
| R3c | |
a = 9.062, c = 11.625
| |
Lattice volume (V)
|
137.8 Å3 |
Formula units (Z)
|
6 |
| tetrahedron | |
| Thermochemistry[1] | |
Std enthalpy of
formation (ΔfH⦵298) |
−146.2 kcal/mol |
| −616.4 kcal/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetracyanomethane or carbon tetracyanide is an organic compound with the chemical formula C(CN)4. It is a percyanoalkane. It is a molecular carbon nitride. The structure can be considered as methane with all hydrogen atoms replaced by cyanide groups. It was first made by Erwin Mayer in 1969.[2][3]
- ^ Barnes, D.S.; Mortimer, C.T.; Mayer, E. (July 1973). "The enthalpy of formation of tetracyanomethane". The Journal of Chemical Thermodynamics. 5 (4): 481–483. doi:10.1016/S0021-9614(73)80095-3.
- ^ Mayer, Erwin (1969). "Darstellung und Eigenschaften von Tetracyanmethan". Monatshefte für Chemie. 100 (2): 462–468. doi:10.1007/BF00904089. S2CID 92450428.
- ^ Britton, D. (1 July 1974). "The crystal structure of tetracyanomethane, C(CN)4". Acta Crystallographica Section B. 30 (7): 1818–1821. Bibcode:1974AcCrB..30.1818B. doi:10.1107/S0567740874005863.