| |||
| Names | |||
|---|---|---|---|
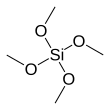
| IUPAC name
Tetramethyl orthosilicate
| |||
| Other names
tetramethyl orthosilicate; methyl silicate; silicic acid, tetramethyl ester; silicon methoxide; TMOS
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.010.598 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SiC4H12O4 | |||
| Molar mass | 152.25 | ||
| Appearance | colourless liquid | ||
| Density | 1.032 | ||
| Melting point | 4 to 5 °C (39 to 41 °F; 277 to 278 K) | ||
| Boiling point | 121 to 122 °C (250 to 252 °F; 394 to 395 K) | ||
| organic solvents | |||
| Vapor pressure | 12 mmHg (25°C)[1] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
toxic | ||
| Flash point | 96 °C; 205 °F; 369 K[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[1] | ||
REL (Recommended)
|
TWA 1 ppm (6 mg/m3)[1] | ||
IDLH (Immediate danger)
|
N.D.[1] | ||
| Related compounds | |||
Other cations
|
Trimethyl borate Trimethyl phosphite | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tetramethyl orthosilicate (TMOS) is the chemical compound with the formula Si(OCH3)4. This molecule consists of four methoxy groups bonded to a silicon atom. The basic properties are similar to the more popular tetraethyl orthosilicate, which is usually preferred because the product of hydrolysis, ethanol, is less toxic than methanol.
Tetramethyl orthosilicate hydrolyzes to SiO2:
In organic synthesis, Si(OCH3)4 has been used to convert ketones and aldehydes to the corresponding ketals and acetals, respectively.[2]
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0428". National Institute for Occupational Safety and Health (NIOSH).
- ^ Sakurai, H. "Silicon(IV) Methoxide" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. doi:10.1002/047084289X.rs012