| |
 Tetramethylammonium ion. Blue: nitrogen, black: carbon, white: hydrogen
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Trimethylmethanaminium chloride | |
| Other names
Tetramethylammonium chloride
Tetramethylazanium chloride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.000.801 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H12NCl | |
| Molar mass | 109.60 g/mol |
| Appearance | White crystals |
| Density | 1.17 g/cm3 |
| Melting point | 425 °C (797 °F; 698 K) (decomposes) |
| Solubility | Soluble in water and methanol. Slightly soluble in ethanol. Insoluble in ether, benzene, chloroform. |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Other anions
|
tetramethylammonium hydroxide |
Other cations
|
tetraethylammonium chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
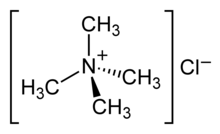
Tetramethylammonium chloride is one of the simplest quaternary ammonium salts, with four methyl groups tetrahedrally attached to the central N. The chemical formula (CH3)4N+Cl− is often abbreviated further as Me4N+Cl−. It is a hygroscopic colourless solid that is soluble in water and polar organic solvents. Tetramethylammonium chloride is a major industrial chemical, being used widely as a chemical reagent[1] and also as a low-residue bactericide in such processes as hydrofracking.[2] In the laboratory, it has fewer synthetic chemical applications than quaternary ammonium salts containing longer N-alkyl substituents, which are used extensively as phase-transfer catalysts.
- ^ "Tetramethylammonium chloride - National Library of Medicine HSDB Database". Archived from the original on 2015-12-22. Retrieved 2012-09-24.
- ^ "What Chemicals Are Used | FracFocus Chemical Disclosure Registry". Archived from the original on 2013-08-14. Retrieved 2012-10-30.