| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetrasulfur tetranitride
| |||
| Systematic IUPAC name
1,3,5,7-tetrathia-2,4,6,8-tetraazacyclooctan-2,4,6,8-tetrayl | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| S4N4 | |||
| Molar mass | 184.287 g/mol | ||
| Appearance | Vivid orange, opaque crystals | ||
| Melting point | 187 °C (369 °F; 460 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
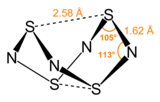
Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque, crystalline explosive is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.[1][2]
Nitrogen and sulfur have similar electronegativities. When the properties of atoms are so highly similar, they often form extensive families of covalently bonded structures and compounds. Indeed, a large number of S-N and S-NH compounds are known with S4N4 as their parent.
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemical Elements (2nd ed.). Boston, MA: Butterworth-Heinemann. pp. 721–725.
- ^ Chivers, T. (2004). A Guide To Chalcogen-Nitrogen Chemistry. Singapore: World Scientific Publishing. ISBN 981-256-095-5.

