| |

| |

| |
| Names | |
|---|---|
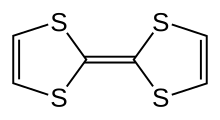
| Preferred IUPAC name
2,2′-Bi(1,3-dithiolylidene) | |
| Other names
Δ2,2-Bi-1,3-dithiole
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1282106 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.045.979 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H4S4 | |
| Molar mass | 204.34 g·mol−1 |
| Appearance | Yellow solid |
| Melting point | 116 to 119 °C (241 to 246 °F; 389 to 392 K) |
| Boiling point | Decomposes |
| Insoluble | |
| Solubility in organic solvents | Soluble[vague] |
| Structure | |
| 0 D | |
| Hazards[1] | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
combustible |
| GHS labelling: | |

| |
| Warning | |
| H317 | |
| P261, P280, P302+P352, P333+P313, P363, P501 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrathiafulvalene (TTF) is an organosulfur compound with the formula (C3H2S2)2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.[2]
- ^ "Tetrathiafulvalene". pubchem.ncbi.nlm.nih.gov.
- ^ Bendikov, M; Wudl, F; Perepichka, D F (2004). "Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics". Chemical Reviews. 104 (11): 4891–4945. doi:10.1021/cr030666m. PMID 15535637.