| |
| Names | |
|---|---|
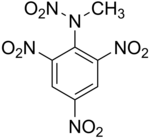
| Preferred IUPAC name
Methyl(2,4,6-trinitrophenyl)nitramide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.848 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 0208 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H5N5O8 | |
| Molar mass | 287.144 g·mol−1 |
| Appearance | Yellow crystalline solid |
| Odor | Odorless |
| Density | 1.73 g/cm3 |
| Melting point | 129.5 °C (265.1 °F; 402.6 K) |
| Boiling point | 187 °C (369 °F; 460 K) decomposes |
| Virtually insoluble | |
| Vapor pressure | <1 mmHg (20°C)[1] |
| Explosive data | |
| Shock sensitivity | Sensitive |
| Friction sensitivity | Sensitive |
| Detonation velocity | 7,570 m/s (24,836 f/s) |
| RE factor | 1.25 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LDLo (lowest published)
|
5000 mg/kg (dog, subcutaneous)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1.5 mg/m3 [skin][1] |
REL (Recommended)
|
TWA 1.5 mg/m3 [skin][1] |
IDLH (Immediate danger)
|
750 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,4,6-Trinitrophenylmethylnitramine or tetryl (C7H5N5O8) is an explosive compound used to make detonators and explosive booster charges.
Tetryl is a nitramine booster explosive, though its use has been largely superseded by RDX. Tetryl is a sensitive secondary high explosive used as a booster, a small charge placed next to the detonator in order to propagate detonation into the main explosive charge.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0607". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Tetryl". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).