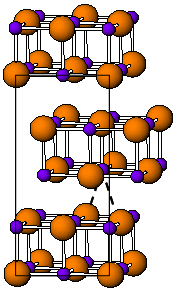
| |
| Names | |
|---|---|
| Other names
Thallium monoiodide
Thallous iodide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.272 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| TlI | |
| Molar mass | 331.287 g/mol[1] |
| Appearance | yellow crystals[1] |
| Density | 7.1 g/cm3[1] |
| Melting point | 441.7 °C (827.1 °F; 714.8 K)[1] |
| Boiling point | 824 °C (1,515 °F; 1,097 K)[1] |
| 0.085 g/L (25 °C)[1] | |
| Solubility | insoluble in alcohol[1] |
| −82.2·10−6 cm3/mol[2] | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Thallium(I) fluoride Thallium(I) chloride Thallium(I) bromide |
Other cations
|
Gallium(I) iodide Indium(I) iodide |
Related compounds
|
Mercury(II) iodide Lead(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thallium(I) iodide is a chemical compound with the formula . It is unusual in being one of the few water-insoluble metal iodides, along with , , , , and .






