 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Tapazole, others |
| Other names | methimazole (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682464 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Protein binding | None |
| Metabolism | Liver |
| Elimination half-life | 5-6 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.439 |
| Chemical and physical data | |
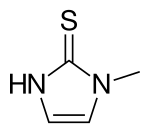
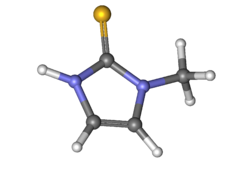
| Formula | C4H6N2S |
| Molar mass | 114.17 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 146 °C (295 °F) |
| Solubility in water | 275[1] mg/mL (20 °C) |
| |
| |
| (verify) | |
Thiamazole, also known as methimazole, is a medication used to treat hyperthyroidism.[2] This includes Graves disease, toxic multinodular goiter, and thyrotoxic crisis.[2] It is taken by mouth.[2] Full effects may take a few weeks to occur.[3]
Common side effects include itchiness, hair loss, nausea, muscle pain, swelling, and abdominal pain.[2] Severe side effects may include low blood cell counts, liver failure, and vasculitis.[2] Use is not recommended during the first trimester of pregnancy due to the risk of congenital anomalies, but it may be used in the second trimester or third trimester.[4] It may be used during breastfeeding.[4] Those who developed significant side effects may also have problems with propylthiouracil.[2] Thiamazole is a cyclic thiourea derivative that works by decreasing the production of thyroid hormones.[2]
Thiamazole was approved for medical use in the United States in 1950.[2] It is on the World Health Organization's List of Essential Medicines.[5][6] It is available as a generic medication.[2] It is also available in Europe and Asia.[7] In 2021, it was the 237th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[8][9]
- ^ "DrugBank: Methimazole (DB00763)". drugbank.ca. Retrieved 21 July 2015.
- ^ a b c d e f g h i "Methimazole Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 8 April 2019.
- ^ Spina D (2008). The Flesh and Bones of Medical Pharmacology E-Book. Elsevier Health Sciences. p. 74. ISBN 9780723437161.
- ^ a b "Methimazole Use During Pregnancy". Drugs.com. Retrieved 8 April 2019.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Yoshihara A, Noh J, Yamaguchi T, Ohye H, Sato S, Sekiya K, et al. (July 2012). "Treatment of graves' disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation". The Journal of Clinical Endocrinology and Metabolism. 97 (7): 2396–2403. doi:10.1186/1756-6614-8-S1-A12. PMC 4480840. PMID 22547422.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Methimazole - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.