| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propane-2-thione[1] | |||
| Systematic IUPAC name
Thiopropan-2-one | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6S | |||
| Molar mass | 74.14 g·mol−1 | ||
| Appearance | Orange to brown liquid | ||
| Odor | Intensely sulfurous, leak-like | ||
| Melting point | −55°C(218.15k/-67°F)[2] | ||
| Boiling point | 70°C(343.15k/158°F)[2] | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Odor, skin irritant | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
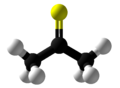
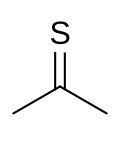
Thioacetone is an organosulfur compound belonging to the -thione group called thioketones with a chemical formula (CH3)2CS. It is an unstable orange or brown substance that can be isolated only at low temperatures.[3] Above −20 °C (−4 °F), thioacetone readily converts to a polymer and a trimer, trithioacetone.[4] It has an extremely potent, unpleasant odor, and is considered one of the worst-smelling chemicals known to humanity.[5]
Thioacetone was first obtained in 1889 by Baumann and Fromm, as a minor impurity in their synthesis of trithioacetone.[2]
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 739. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ a b c William H. Sharkey (1979): "Polymerization through the carbon-sulfur double bond". Polymerization, series Advances in Polymer Science, volume 17, pages 73–103. doi:10.1007/3-540-07111-3_2
- ^ V.C.E. Burnop; K.G. Latham (1967). "Polythioacetone Polymer". Polymer. 8: 589–607. doi:10.1016/0032-3861(67)90069-9.
- ^ R.D. Lipscomb; W.H. Sharkey (1970). "Characterization and polymerization of thioacetone". Journal of Polymer Science Part A: Polymer Chemistry. 8 (8): 2187–2196. Bibcode:1970JPoSA...8.2187L. doi:10.1002/pol.1970.150080826.
- ^ Cite error: The named reference
Derek Lowewas invoked but never defined (see the help page).