| |||
| Clinical data | |||
|---|---|---|---|
| Trade names | Tepadina | ||
| AHFS/Drugs.com | Monograph | ||
| MedlinePlus | a682821 | ||
| License data | |||
| Pregnancy category |
| ||
| Routes of administration | Intravenous, intracavitary, intravesical | ||
| ATC code | |||
| Legal status | |||
| Legal status | |||
| Pharmacokinetic data | |||
| Metabolism | Liver (CYP2B6, CYP3A) | ||
| Elimination half-life | 1.5–4.1 hours | ||
| Excretion | Kidney 6 hours for thiotepa 8 hours for TEPA | ||
| Identifiers | |||
| |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.000.124 | ||
| Chemical and physical data | |||
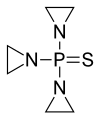
| Formula | C6H12N3PS | ||
| Molar mass | 189.22 g·mol−1 | ||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Thiotepa (INN[8]), sold under the brand name Tepadina among others, is an anti-cancer medication.[5][7][9]
Thiotepa is an organophosphorus compound with the formula (C2H4N)3PS.[10]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Tepadina (Link Medical Products Pty Ltd T/A Link Pharmaceuticals)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 18 March 2023. Retrieved 29 April 2023.
- ^ "Cancer therapies". Health Canada. 8 May 2018. Retrieved 13 April 2024.
- ^ "Thiotepa 100 mg powder for concentrate for solution for infusion". (emc). 27 October 2022. Retrieved 14 August 2024.
- ^ a b "Tepadina- thiotepa injection, powder, for solution". DailyMed. Archived from the original on 12 August 2021. Retrieved 11 August 2021.
- ^ "Highlights of prescribing information" (PDF). www.accessdata.fda.gov.
- ^ a b "Tepadina EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 6 March 2021. Retrieved 30 April 2021.
- ^ "International Non-Proprietary Names for Pharmaceutical Preparations. Recommended International Non-Proprietary Names (Rec. I.N.N.): List 4" (PDF). World Health Organization. March 1962. p. 111. Archived from the original (PDF) on 18 May 2016. Retrieved 27 November 2016.
- ^ Cite error: The named reference
FDAwas invoked but never defined (see the help page). - ^ Maanen MJ, Smeets CJ, Beijnen JH (August 2000). "Chemistry, pharmacology and pharmacokinetics of N,N',N" -triethylenethiophosphoramide (ThioTEPA)". Cancer Treatment Reviews. 26 (4): 257–68. doi:10.1053/ctrv.2000.0170. PMID 10913381.