| |
| Names | |
|---|---|
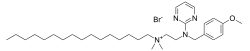
| Preferred IUPAC name
N-(2-{[(4-Methoxyphenyl)methyl](pyrimidin-2-yl)amino}ethyl)-N,N-dimethylhexadecan-1-aminium bromide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.212 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C32H55BrN4O | |
| Molar mass | 591.723 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thonzonium bromide is a monocationic detergent.[1] A solution of it is thus a surfactant and a detergent that promotes tissue contact by dispersion and penetration of the cellular debris and exudate of the containing solution.[2]
It is used in cortisporin-TC ear drops to help penetration of active ingredients through cellular debris for its antibacterial action.[3][4]
- ^ "Thonzonium bromide". Long Island University. Retrieved 2023-01-02.
- ^ "Otic Antibiotics Review 2009" (PDF). Otic Antibiotics Review. 6 (1): 11. 2009-12-15. Archived (PDF) from the original on 2023-01-03. Retrieved 2023-01-03 – via Nevada Department of Health and Human Services.
- ^ "Cortisporin-TC: Indications, Side Effects, Warnings". Drugs.com. 2022-10-15. Archived from the original on 2023-01-03. Retrieved 2023-01-03.
- ^ "CORTISPORIN-TC- colistin sulfate, neomycin sulfate, thonzonium bromide and hydrocortisone acetate suspension" (PDF). Food and Drug Administration. 2019. Archived (PDF) from the original on 2023-01-03. Retrieved 2023-01-02.