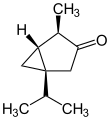
| |||
 Ball-and-stick model of (−)-α-thujone[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
α: (1S,4R,5R)-4-Methyl-1-(propan-2-yl)bicyclo[3.1.0]hexan-3-one
β: (1S,4S,5R)-4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one | |||
| Other names
Bicyclo[3.1.0]hexan-3-one, 4-methyl-1-(1-methylethyl)-, [1S-(1α,4α,5α)]-
α-Thujone β-Thujone Thujone, cis 3-Thujanone, (1S,4R,5R)-(−)- Thujon 3-Thujanone, (−)- l-Thujone; 4-Methyl-1-(1-methylethyl)bicyclo[3.1.0]hexan-3-one-, (1S,4R,5R)- 3-Thujone; cis-Thujone (Z)-Thujone (−)-Thujone; Bicyclo(3.1.0)hexan-3-one, 4-methyl-1-(1-methylethyl)-, (1S,4R,5R)- NSC 93742 1-isopropyl-4-methylbicyclo[3.1.0]hexan-3-one | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| 4660369 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.096 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16O | |||
| Molar mass | 152.237 g·mol−1 | ||
| Density | 0.92 g/cm3 (β-thujone); 0.9116 g/cm3 (α-thujone) | ||
| Melting point | <25 °C | ||
| Boiling point | 203 °C (397 °F; 476 K) (alpha,beta-thujone) | ||
| 407 mg/L | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H302 | |||
| P264, P270, P301+P312, P330, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Thujone (/ˈθuːdʒoʊn/ [2]) is a ketone and a monoterpene that occurs predominantly in two diastereomeric (epimeric) forms: (−)-α-thujone and (+)-β-thujone.[3][4]
Though it is best known as a chemical compound in the spirit absinthe, it is unlikely to be responsible for absinthe's alleged stimulant and psychoactive effects due to the small quantities present.[5][6][7]
Thujone acts on the GABAA receptor as an antagonist. As a competitive antagonist of GABAA receptor, thujone alone is considered to be convulsant,[8] though by interfering with the inhibitory transmitter GABA, it may convey stimulating, mood-elevating effects at low doses.[citation needed] It is also found in perfumery as a component of several essential oils.[citation needed]
In addition to the naturally occurring (−)-α-thujone and (+)-β-thujone, two other forms are possible: (+)-α-thujone and (−)-β-thujone. In 2016, they were found in nature as well,[9] in Salvia officinalis.
-
(−)-α-thujone
-
(+)-α-thujone
-
(+)-β-thujone
-
(−)-β-thujone
- ^ Richert, Clemens; Krupp, Felix; Frey, Wolfgang (2020). "Absolute Configuration of Small Molecules by Co-Crystallization". Angew. Chem. Int. Ed. 59 (37): 15875–15879. doi:10.1002/anie.202004992. PMC 7540501. PMID 32441841.
- ^ Derived from the Ancient Greek θυία, thuj(a), a kind of cedar + -ωνη, -one, feminine patronymic for a chemical relative of acetone
- ^ Perry NB, Anderson RE, Brennan NJ, Douglas MH, Heaney AJ, McGimpsey JA, Smallfield BM (1999). "Essential Oils from Dalmatian Sage (Salvia officinalis L.): Variations among Individuals, Plant Parts, Seasons, and Sites". J. Agric. Food Chem. 47 (5): 2048–2054. Bibcode:1999JAFC...47.2048P. doi:10.1021/jf981170m. PMID 10552494.
- ^ Oppolzer W, Pimm A, Stammen B, Hume WE (1997). "Palladium-Catalysed Intramolecular Cyclisations of Olefinic Propargylic Carbonates and application to the diastereoselective synthesis of enantiomerically pure (−)-α-thujone". Helv. Chim. Acta. 80 (3): 623–639. doi:10.1002/hlca.19970800302.
- ^ Dettling A, Grass H, Schuff A, Skopp G, Strohbeck-Kuehner P, Haffner HT (2004). "Absinthe: attention performance and mood under the influence of thujone". J. Stud. Alcohol. 65 (5): 573–81. doi:10.15288/jsa.2004.65.573. PMID 15536765.
- ^ Absinthe Myths Finally Laid To Rest
- ^ Chemical Composition of Vintage Preban Absinthe with Special Reference to Thujone, Fenchone, Pinocamphone, Methanol, Copper, and Antimony Concentrations
- ^ Olsen, Richard W. (2000-04-25). "Absinthe and γ-aminobutyric acid receptors". Proceedings of the National Academy of Sciences of the United States of America. 97 (9): 4417–4418. Bibcode:2000PNAS...97.4417O. doi:10.1073/pnas.97.9.4417. ISSN 0027-8424. PMC 34311. PMID 10781032.
- ^ Williams, Jack D.; Yazarians, Jessica A.; Almeyda, Chelcie C.; Anderson, Kristin A.; Boyce, Gregory R. (23 May 2016). "Detection of the Previously Unobserved Stereoisomers of Thujone in the Essential Oil and Consumable Products of Sage (Salvia officinalis L.) Using Headspace Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry". Journal of Agricultural and Food Chemistry. 64 (21): 4319–4326. Bibcode:2016JAFC...64.4319W. doi:10.1021/acs.jafc.6b01065. PMID 27181395.